Feb 19 2020
At the Tokyo Institute of Technology (Tokyo Tech), researchers have developed the world’s first visible-light photoelectrochemical system for a water-splitting process using cobalt-doped titanium dioxide (TiO2). Cobalt is a material that occurs abundantly on Earth.
 Cheap and easy enhancement of photoanodes for water oxidation. Image Credit: Tokyo Institute of Technology.
Cheap and easy enhancement of photoanodes for water oxidation. Image Credit: Tokyo Institute of Technology.
The presented technique is easy and represents a major advancement toward the search for a low-cost water splitting process to generate hydrogen, which is known to be a clean substitute for fossil fuel.
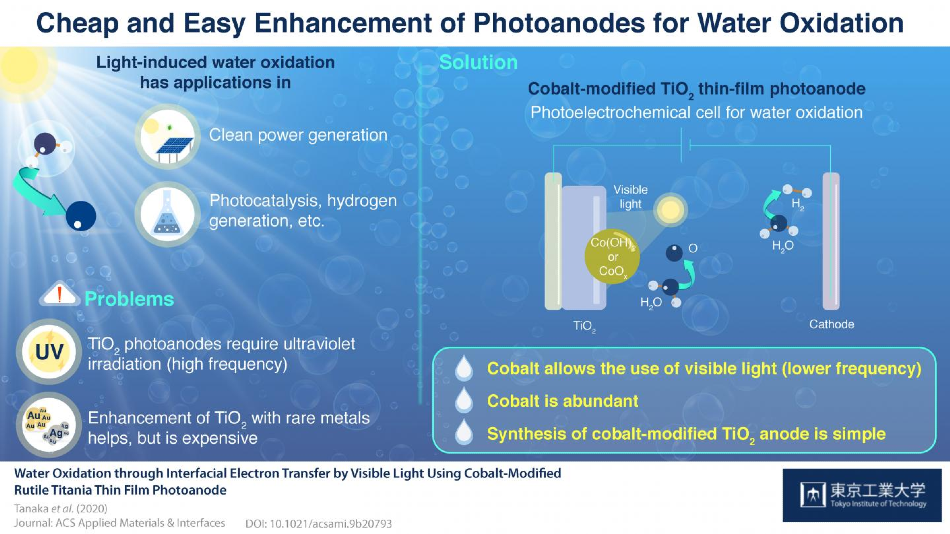
Photoelectrochemical water splitting is a process that involves the use of light energy to split water molecules into oxygen (O2) and hydrogen (H2). It offers a potential method to acquire pure hydrogen that can be used as an alternative clean fuel.
Photoelectrochemical water splitting is performed in electrochemical cells containing a cathode and an anode immersed in water; both anode and anode are linked via an external circuit.
Water oxidation takes place at the anode, whereby O2 is generated by harvesting energy from light waves. These light waves supply energy to the electrons present in the anode material and enable these electrons to travel via the external circuit to reach the cathode material. Here, the cathode material and the received electrons lead to the formation of H2.
So far, finding photoelectrochemical systems that efficiently perform this process has been very difficult due to many reasons. TiO2 is a popular and extensively used photoanode material. But this material can only absorb high-energy light—that is, energy from light in the ultraviolet region.
Since it is more desirable to manipulate the energy from longer-wavelength light, TiO2 can be combined with noble metals such as silver or gold, to make it sensitive to visible light. But this approach would prove very costly in terms of commercial applications.
To resolve this challenge, a team of researchers from Tokyo Tech developed the world’s first visible-light photoanode made of cobalt-doped TiO2. Cobalt is an earth-abundant material.
The researchers’ study was published in the ACS Applied Materials & Interfaces journal. The study describes the process of photoanode fabrication, which is unexpectedly simple. In this process, thin TiO2 films are produced onto a substrate using a typical procedure, and cobalt is subsequently introduced by submerging the films into a solution containing aqueous cobalt nitrate.
This study demonstrates that a visible light-driven photoelectrochemical cell for water oxidation can be constructed through the use of earth-abundant metals without the need for complicated preparation procedures.
Kazuhiko Maeda, Study Lead and Professor, Department of Chemistry, Tokyo Institute of Technology
Using numerous types of scanning electron microscopy and spectrometry analyses, the scientists discovered the particular structure and composition of the cobalt-altered surface of the TiO2 photoanode to find out how cobalt enables the TiO2 material to absorb visible light to move electrons and promote the oxidation of water.
It was found that in addition to capturing visible light and transferring charges (electrons) at the TiO2 interface, cobalt domains also act as catalytic sites that cause the oxidation of water. The scientists also discovered that the performance of the final modified photoanode is affected by the structure of the base TiO2 thin film, apparently by enabling a better or worse accommodation of the atoms of cobalt.
The fabrication parameters can be modified to easily alter the TiO2 film structure. This technique enabled the researchers to perform various tests to get a better understanding of this phenomenon.
Despite this, more studies still need to be carried out because the design of the photoanode will have to be further improved to enhance the charge transfer process, which takes place between the TiO2 substrate and the cobalt atoms, to obtain higher water oxidation rates.
Nonetheless, the recommended water oxidation system is non-sacrificial, which provides a major benefit. To put this in simpler terms, the materials used do not depend on energy-rich oxidants, reductants, or both (that is, sacrificial reagents).
So far, cobalt-sensitized water photooxidation systems had been comprised of powder-based photocatalysis, which work only in the presence of a sacrificial electron acceptor. Therefore, the present study also demonstrates sacrificial reagent-free visible-light water splitting using a cobalt-sensitized semiconductor material (TiO2).
Kazuhiko Maeda, Study Lead and Professor, Department of Chemistry, Tokyo Institute of Technology
It is hoped that the study will act as a step forward to all those researchers who are struggling to realize low-cost water splitting to guarantee a greener future.