Apr 27 2020
At the Tokyo Institute of Technology, researchers have designed an enhanced catalyst by using calcium hydride—the common dehydrating agent—and adding fluoride to it. The catalyst simplifies the synthesis of ammonia at just 50 °C and uses only half the energy needed in the current methods.
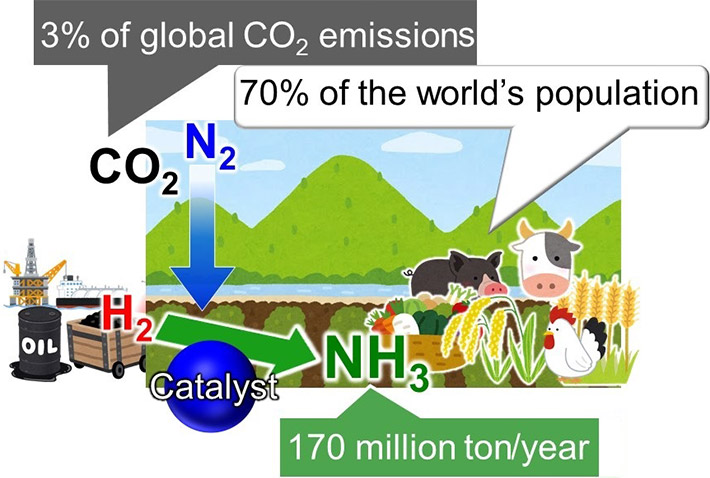 The ammonia challenges. Ammonia (NH3) is one of the most important industrial chemicals today, synthesized globally for use in fertilizers that then enable food production for approximately 70% of the world’s population. Ammonia is currently obtained by reacting nitrogen (N2) from air with hydrogen (H2). This reaction requires high energy and is, therefore, powered by fossil fuels, contributing to over 3% of the global CO2 emissions Image Credit: Tokyo Institute of Technology.
The ammonia challenges. Ammonia (NH3) is one of the most important industrial chemicals today, synthesized globally for use in fertilizers that then enable food production for approximately 70% of the world’s population. Ammonia is currently obtained by reacting nitrogen (N2) from air with hydrogen (H2). This reaction requires high energy and is, therefore, powered by fossil fuels, contributing to over 3% of the global CO2 emissions Image Credit: Tokyo Institute of Technology.
This method paves the way for synthesizing ammonia with lower greenhouse gas emissions and low energy consumption.
Ammonia is crucial for manufacturing plant fertilizer, which in turn feeds nearly 70% of the world’s population. In the industrial sector, the Haber-Bosch process is used to synthesize ammonia. The process involves first making methane to react with steam to generate hydrogen (H2), and then making hydrogen to react with nitrogen to synthesize ammonia (NH3).
The disadvantage of this process is that if there is an increase in temperature, the yield reduces. To obtain a good yield constantly, it is essential to increase the pressure applied in the reaction chamber, which needs high energy.
Moreover, the iron-based catalysts that are utilized for the reaction are effective only above 350 °C. To maintain such high temperatures, a considerable amount of energy is needed. Above all, the yield produced is only 30% to 40%.
At present, fossil fuels are utilized to power the process, releasing huge quantities of carbon dioxide into the air. Although alternative renewable resources, like wind energy, have been employed, those have not been sustainable.
The reaction should occur at low temperatures to enhance the yield and decrease the risk to the surroundings. To this end, catalysts that allow reaction at low temperatures are needed. Until now, these catalysts have been unachievable for researchers.
Conventional catalysts lose the catalytic activity for ammonia formation from N2 and H2 gases at 100-200 °C, even if they exhibit high catalytic performance at high temperatures.
Researchers, Tokyo Institute of Technology
The researchers seem to have solved the issue regarding the catalyst. Under the guidance of Dr Michikazu Hara, the researchers have designed a catalyst that is effective even at 50 °C.
Our catalyst produces ammonia from N2 and H2 gases at 50 °C with an extremely small activation energy of 20 kJ mol−1, which is less than half that reported for conventional catalysts.
Dr Michikazu Hara and colleagues, Tokyo Institute of Technology
The study has been described in a paper published in the Nature Communications journal.
The new catalyst includes a strong CaFH solution, where ruthenium (Ru) nanoparticles are deposited on its surface. The catalyst is made effective at lower pressures and temperatures by the addition of fluoride (F−) to calcium hydride (CaH2), which is a common dehydrating agent.
Following computational and spectroscopic analyses, the researchers put forward a probable mechanism through which the catalyst enables the production of ammonia.
The calcium–fluoride (Ca–F) bond is more powerful compared to the calcium–hydrogen (Ca–H) bond. The existence of the Ca–F bond disables the Ca–H bond, and the Ru can extract H2 atoms from the catalyst crystal, which leaves back the electrons in their place. Then, the H atoms desorb from the Ru nanoparticles in the form of H2 gas.
This process takes place even at 50 °C. The consequent charge repulsion between the F− ions and trapped electrons in the crystal reduces the energy barriers for these electrons to discharge, thereby offering the material high electron-donating ability.
Such discharged electrons strike the bonds between the nitrogen atoms in the N2 gas, thus enabling ammonia production.
This novel technique utilized in the production of ammonia reduces the energy requirements, thus decreasing the emissions of carbon dioxide caused by the use of huge amounts of fossil fuels. The outcomes of this research point toward the chances of an ecologically sustainable Haber-Bosch process, paving the way to the next revolution in the production of agricultural foods.