May 4 2020
A portable and more eco-friendly way to synthesize hydrogen peroxide, a disinfectant in demand, could now allow hospitals to produce their own supply.
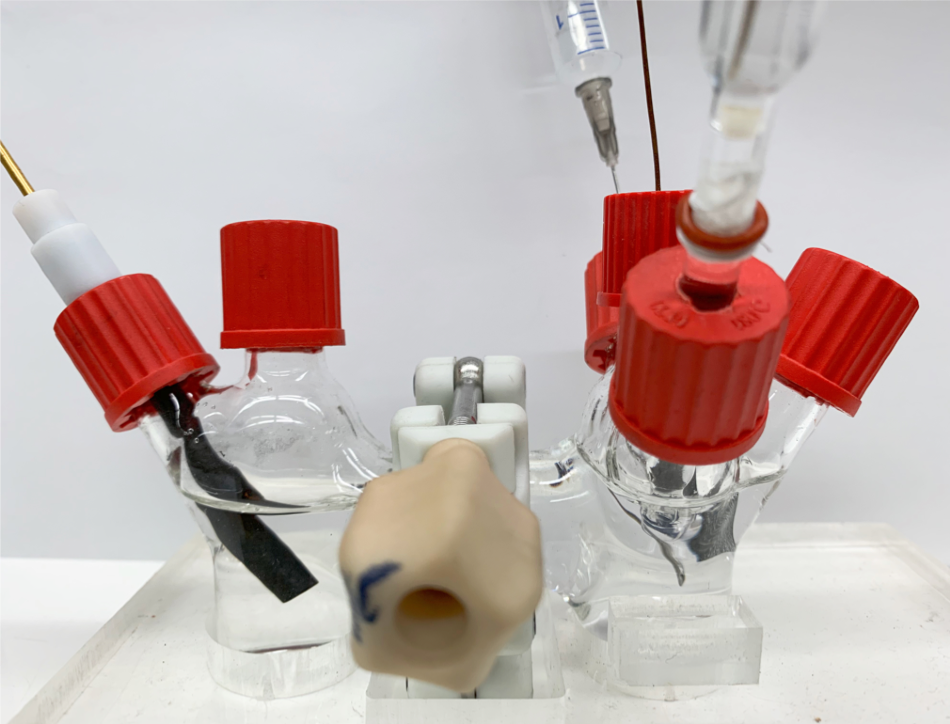 The researchers’ H-cell setup used in developing their hydrogen peroxide production method. Image Credit: Zheng Chen Lab.
The researchers’ H-cell setup used in developing their hydrogen peroxide production method. Image Credit: Zheng Chen Lab.
The study, which is a joint effort between the University of California San Diego, Brookhaven National Laboratory, Columbia University, the University of Calgary, and the University of California, Irvine, has been reported in a paper published in the Nature Communications journal.
Hydrogen peroxide recently hit the headlines since medical centers and researchers across the United States have been testing its potential use to disinfect N95 masks to handle the shortage of masks due to the COVID-19 pandemic.
Although the results that have been achieved until now are encouraging, some researchers fear that the poor shelf life of the chemical could make these decontamination efforts expensive.
The major issue is that hydrogen peroxide is not stable—it begins to disintegrate into oxygen and water even before the bottle is opened. Moreover, it disintegrates even more quickly as soon as it is exposed to light or air.
You maybe only have just a couple of months to use it before it expires, so you would have to order batches more frequently to keep a fresh supply. And because it decomposes so quickly, shipping and storing it become very expensive.
Zheng Chen, Nanoengineering Professor, University of California San Diego
Chen and his team designed an easy, cost-effective, and rapid way to produce hydrogen peroxide in house by simply utilizing a small flask, a catalyst, air, electricity, and an off-the-shelf electrolyte.
Our goal is to create a portable setup that can be simply plugged in so that hospitals, and even households, have a way to generate hydrogen peroxide on demand. No need to ship it, no need to store it, and no rush to use it all before it expires. This could save up to 50 to 70% in costs.
Zheng Chen, Nanoengineering Professor, University of California San Diego
One more benefit of this technique is that it is less toxic compared to the industrial processes.
Furthermore, the technique is based on a chemical reaction where one oxygen molecule combines with two protons and two electrons in an acidic electrolyte solution to generate hydrogen peroxide.
This kind of reaction is called the two-electron oxygen reduction reaction, and it is easy-to-use since it has the ability to generate dilute hydrogen peroxide with the preferred concentration as required.
In the next step, we will develop electrocatalysts suitable for other electrolyte solutions to further increase the range of its applications.
Qiaowan Chang, Graduate Student, Chemical Engineering, University of California San Diego
The group has designed a unique catalyst, which is key to make this reaction feasible. The catalyst is composed of partially oxidized carbon nanotubes, signifying that the surface includes oxygen atoms.
The oxygen atoms are linked to small clusters consisting of three to four palladium atoms. Such bonds between the oxygen atoms and palladium clusters are the ones that allow the reaction to take place with high activity and selectivity owing to the maximum binding energy of the essential intermediate at the time of the reaction.
According to Jingguang Chen, a chemical engineering professor at Columbia University, “The coordination between oxygen-modified Pd cluster and the oxygen-containing functional groups on carbon nanotubes is the key to enhancing its catalytic performance.”
Initially, the researchers designed this technique to make the processes of battery recycling greener. Hydrogen peroxide is one of the chemicals employed to derive and retrieve metals such as nickel, copper, magnesium, and cobalt from used lithium-ion batteries.
In the same way, this technique also makes the activation of hydrocarbon molecules more effective, which is a crucial step in several industrial chemical processes.
Chen stated, “We had been working on this project for about one and a half years. As we were wrapping things up, the COVID-19 pandemic hit.”
The team was highly motivated to pivot directions by the news reports regarding the use of hydrogen peroxide vapor to decontaminate N95 masks for reuse.
“We saw that there was a more pressing need for efforts to help health care workers who may not have sufficient protection while caring for patients suffering from the new coronavirus,” added Chen.
The study is in the proof-of-concept phase. Proceeding further, the researchers will strive to enhance and scale up the technique for possible use in hospitals. The forthcoming studies include altering the technique to use a neutral electrolyte (essentially a salt solution) rather than an acidic one, which would be ideal for clinical and household applications, said Chen.
Currently, a part of this ongoing work is financially supported by the UC San Diego’s Sustainable Power and Energy Center.
This study was also partially funded by the ACS Petroleum Research Fund (59989-DNI5), the U.S. Department of Energy (DE-FG02-13ER16381), and the UC San Diego Jacobs School of Engineering.
Journal Reference:
Chang, Q., et al. (2020) Promoting H2O2 production via 2-electron oxygen reduction by coordinating partially oxidized Pd with defect carbon. Nature Communications. doi.org/10.1038/s41467-020-15843-3.