Jun 8 2020
Researchers from the University of Michigan, Purdue University, and the University of Liverpool in the United Kingdom have discovered how to measure the number of “hot charge carriers” (for instance, electrons with extra energy) in a metal nanostructure.
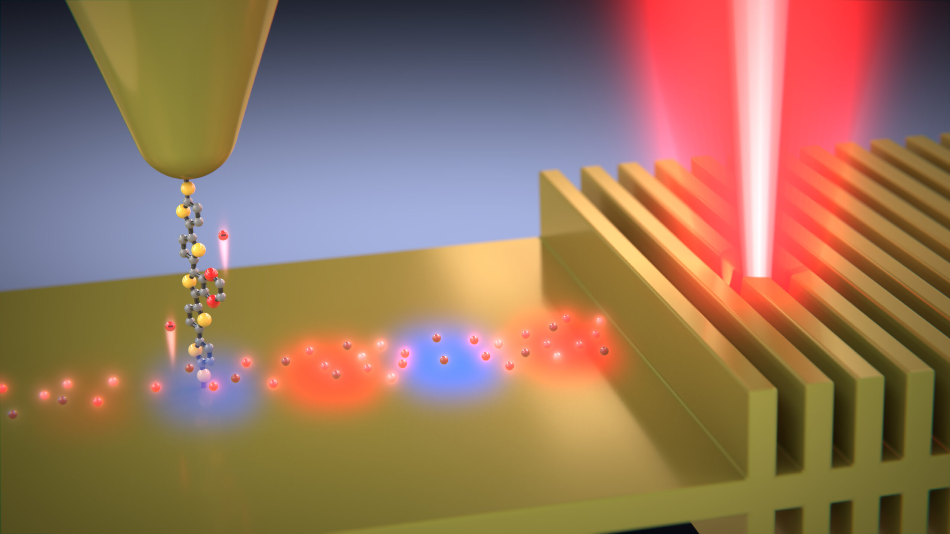 Hot electrons travel along the molecule into the probe tip. The molecule only allows electrons within a narrow range of energies to pass. Image Credit: Enrique Shagun, Scixel.
Hot electrons travel along the molecule into the probe tip. The molecule only allows electrons within a narrow range of energies to pass. Image Credit: Enrique Shagun, Scixel.
The study findings could help answer a key question related to technologies such as energy conversion.
For example, if you wanted to employ light to split water into hydrogen and oxygen, you can use hot charge carriers because electrons that are more energetic can more readily participate in the reaction and drive the reaction faster. That’s one possible use for hot carriers in energy conversion or storage applications.
Edgar Meyhofer, Professor of Mechanical Engineering, University of Michigan
The study was co-led by Meyhofer and Vladimir Shalaev, a professor of electrical and computer engineering who led the contribution from Purdue.
In addition, the study outcomes show the higher efficiency of thinner metals in using light to produce hot charge carriers. The motion of electrons on the surfaces of materials such as silver and gold can be drive by using light, thus forming waves called surface plasmons. These waves, in turn, can produce hot charge carriers.
The general distribution of charge carrier energies was compared with air at room temperature. All molecules in the air do not have the same energy—their average energy is governed by the temperature.
In general, within a material, the energies of positively charged holes and negatively charged electrons follow similar distributions. However, in the case of materials that support surface plasmons, some charge carriers can be given additional energy by using light as though the material were considerably hotter—over 2000 °F.
The hot charge carriers were generated by shining laser light onto a gold film with a thickness of just 13 nm, or about a hundred gold atoms thick. The gold film included minuscule ridges spaced such that they would resonate along with the laser light and produce the surface plasmon waves.
The energies of the charge carriers were then measured by drawing them into a gold electrode—a scanning tunneling microscope’s tip—through gatekeeper molecules.
The gatekeeper molecules produced by the Liverpool researchers in collaboration with a private company were key to the experiment. The molecules permit only charge carriers with specific energies to pass. The researchers repeated the experiments with different molecules to find out the energy distribution of the charge carriers.
Electrons can be thought of as cars running at different speeds on a highway. The molecule acts like an operator—it only allows cars travelling at a certain speed to pass through.
Kun Wang, Postdoctoral Fellow, University of Michigan
In addition, the researchers compare the molecules to a prism that separates the spectrum of electron energies instead of the colors in light.
Wang, who is a postdoctoral fellow in Meyhofer’s group, worked with Harsha Reddy, a PhD student in electrical and computer engineering at Purdue, for over 18 months on how to make this concept work.
“This idea of molecular filters was something no one else in the field has realized in the past,” stated Reddy, who works in Shalaev’s lab.
Once Wang and Reddy created a successful technique, they repeated the experiments using a second gold structure, which is about 6 nm thick. This structure was more efficient at producing hot charge carriers than the 13-nm version.
This multidisciplinary basic research effort sheds light on a unique way to measure the energy of charge carriers. These results are expected to play a crucial role in developing future applications in energy conversion and photocatalysis and photodetectors, for instance, that are of great interest to the Department of Defense.
Chakrapani Varanasi, Program Manager, Army Research Office
The team’s Multidisciplinary University Research Initiative was funded by the Army Research Office.
The researchers have now demonstrated the method and believe that it can be used by others to investigate and optimize nanostructures. This is crucial for applications like conversion of sunlight into chemical energy since the number of hot charge carriers has an impact on how well a catalyst can direct light energy to a chemical reaction.
Additional funding for the study was provided by the Department of Energy and the Office of Naval Research. Complementary calculations were supported by seed funding from the U-M Department of Mechanical Engineering.
Journal Reference:
Reddy, H., et al. (2020) Determining plasmonic hot-carrier energy distributions via single-molecule transport measurements. Science. doi.org/10.1126/science.abb3457.