Sep 10 2020
Bacteria and iron minerals can be the primary agents that cause the emission of carbon dioxide (CO2) from the soil.
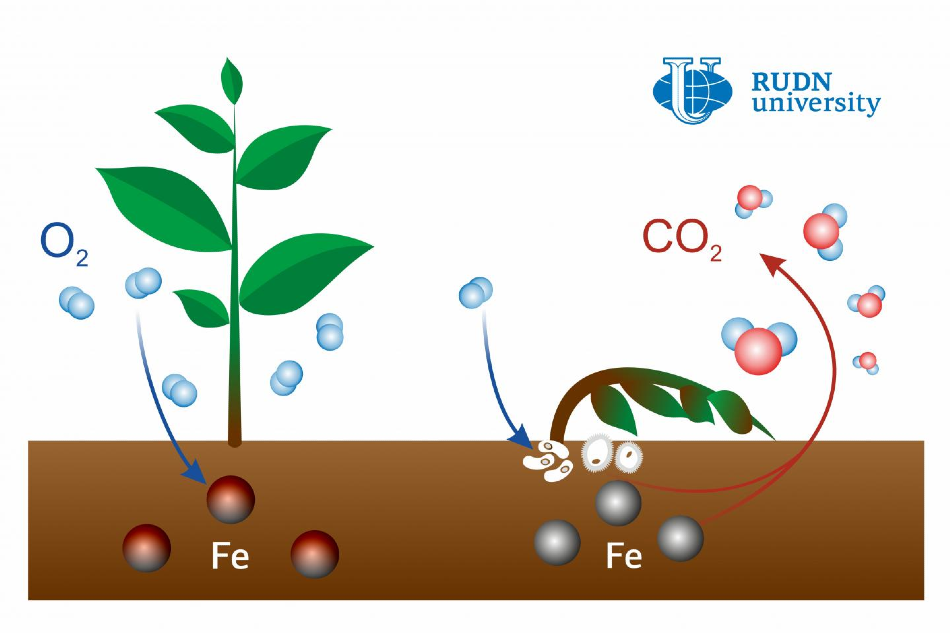
Image Credit: RUDN University.
This conclusion was made by a soil scientist from RUDN University after examining the process of micro-level decomposition of organic plant waste.
Hydrogen peroxide and iron enter into a reaction, which leads to the formation of oxygen radicals or active oxygen forms. These radicals ruin plant waste present in the soil and support the emission of carbon dioxide. The study results were published in the Geoderma journal.
CO2 is believed to be a major reason for global warming, and nearly 50% of this gas is discharged into the air from the soil. Decomposing plant waste is often present in the most active “soil breathing” areas.
These regions tend to have hotspots—that is, local zones measuring up to 1 cm3 in volume in which the decomposition process is faster by almost 100 times. Because of a combination of good aeration and high moisture, such hotspots provide ideal living conditions to soil microbes.
Earlier, microbial activity had been believed to be the reason for the emission of active CO2. But the soil scientist from RUDN University has confirmed that this is actually caused by the oxidation-reduction transitions of soil iron.
Previously we used to believe that the reason for carbon dioxide emissions from hotspots were microorganisms that treated plant waste with special enzymes and turned it into gas. However, we demonstrated that a big part of this process was due not only to enzymes. Iron facilitates the formation of active oxygen forms (radicals) that affect insoluble organic matter, destroy it, and turn it into the soluble state.
Yakov Kuzyakov, PhD in Biology and Head of the Center for Mathematical Modeling and Design of Sustainable Ecosystems, RUDN University
The researcher also emphasized that microbes expedite the decomposition of plant waste only a few times, and not hundreds of times.
During their activity, microorganisms discharge hydrogen peroxide that can react with iron and take a single electron from it, producing active oxygen forms, that is, substances containing one free unpaired electron.
This kind of oxygen is known to be chemically active and rapidly oxidizes organic matter, leading to its destruction.
To verify that active oxygen forms are responsible for causing the emission of CO2, the group produced an artificial hotspot. To achieve this, they placed 300 g of soil in a container, introduced plant waste (straw), and also a solution of iron sulfate.
The level of moisture in the container was maintained at 90%. The researchers quantified the amount of CO2 released from the container for 40 days and subsequently compared the findings to the measurement data gathered from soils, without straw and iron, and with low (45%) levels of moisture.
Within the container, the sample emitted 22 mg of CO2 for every 1 kg of soil, which was around 22 times more than the amount discharged by low-moisture soil and by soil without plant waste.
Following that, the researchers quantified the variations in the volume of active oxygen forms, carbon, hydrogen peroxide, and iron in the vicinity of the hotspot. Depending on the ratio of all these substances, the researchers concluded that iron generated active oxygen by reacting with hydrogen peroxide.
Unlike earlier theories, ours is focused on the primary role of active oxygen forms. It is a simple scenario. At first, plant waste stimulates the growth of bacteria. When there are enough bacteria, they consume almost all oxygen.
Yakov Kuzyakov, PhD in Biology and Head of the Center for Mathematical Modeling and Design of Sustainable Ecosystems, RUDN University
Kuzyakov added, “These are perfect conditions for the concentration of iron which otherwise would oxidize. Then the iron reacts with hydrogen peroxide, and organic matter is decomposed under the influence of active oxygen forms. In its decomposed state it attracts even more bacteria, and the process intensifies several times.”
The research work was the first one to verify that biological processes that occur in soil hotspots are fueled by the activity of free radicals (that is, substances with a free electron).
The team believes that this mechanism may also occur in other hotspots, for instance, those found in the rhizosphere (the soil around plant roots). In the days to come, this data can be used to decrease the emission of CO2 from the soil.
Journal Reference:
Du, H.-Y., et al. (2020) An iron-dependent burst of hydroxyl radicals stimulates straw decomposition and CO2 emission from soil hotspots: Consequences of Fenton or Fenton-like reactions. Geoderma. doi.org/10.1016/j.geoderma.2020.114512.