Apr 16 2021
A new chemical process synthesizes useful, biodegradable chemicals, which can be used as detergents and surfactants in a wide variety of applications, from disposed plastics.
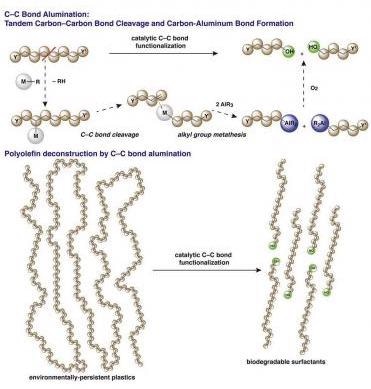 Long hydrocarbon chains of polymers are broken into shorter units with the introduction of aluminum end groups. Image Credit: AMES Laboratory.
Long hydrocarbon chains of polymers are broken into shorter units with the introduction of aluminum end groups. Image Credit: AMES Laboratory.
The process, which was discovered by researchers from the Institute for Cooperative Upcycling of Plastics (iCOUP), an Energy Frontier Research Center led by Ames Laboratory, has the ability to make more sustainable and economically beneficial lifecycles for plastics.
The focus of the study was to disintegrate polyolefins, which constitute over half of all disposed plastics and consists of almost every kind of product that can be imagined—pipe systems, food packaging, toys, fabrics, water bottles, furniture, cars and shoes.
Plastics, and especially polyolefins, are materials you could call too successful. They are fantastic—strong, lightweight, thermally stable, chemically resistant—for all the applications that we use them for, but the problem comes when we don’t need them anymore.
Aaron Sadow, Director, Institute for Cooperative Upcycling of Plastics
The chemical structure of polyolefin plastics makes them very hard and strong—long strong chains of carbon-carbon bonds. This also makes them tough to disintegrate. Moreover, polyolefins normally do not have the chemical groups that can be targeted during the disintegration processes.
Several existing processes for recycling plastic lead to less usable, less valuable components, which the economic viability of recycling much less appealing.
The new process involves using what science already knows regarding the main steps of polymerization—the collecting of long polymer strands—but the other way around. That is, it involves breaking a few of the carbon-carbon bonds in the chains.
As soon as some carbon-carbon bonds are broken, the short polymer chains shift to an aluminum end group to develop reactive species.
For this new process, the catalysts and reactions are associated with those employed in alkene polymerization, thereby benefiting from clearly understood catalytic chemistry.
Lastly, the intermediates of this latest transformation have been easily transformed into fatty acids or fatty alcohols, or utilized in other synthetic chemistry, to make materials or chemicals that are precious in a range of ways—as cosmetics, pharmaceuticals, emulsifiers, and detergents.
Since the process is regulated catalytically, preferred product chain lengths can be aimed for synthesis.
The finest aspect of the process is that its end products are biodegradable, in contrast to polypropylene and polyethylene starting materials.
Fatty acids and alcohols biodegrade in the environment relatively quickly. If these byproducts go on to find a new use elsewhere, that’s wonderful, but it also has an end of life, which means it won’t accumulate in the environment as plastics have.
Aaron Sadow, Director, Institute for Cooperative Upcycling of Plastics
Journal Reference:
Kanbur, U., et al. (2021) Catalytic carbon–carbon bond cleavage and carbon-element bond formation give new life for polyolefins as biodegradable surfactants. Chem. doi.org/10.1016/j.chempr.2021.03.007.