Apr 21 2021
Even though nitrous oxide (N2O) is a greenhouse gas that greatly contributes to global warming, gas separation technology has mostly focused on carbon dioxide. Now, scientists from Korea Maritime and Ocean University are exploring a new concept for N2O separation using an organic compound called hydroquinone clathrate. They found that this compound can selectively trap either N2O or nitrogen gas (N2) depending on its crystalline structure, paving the way for a cost-effective N2O/N2 separation technology.
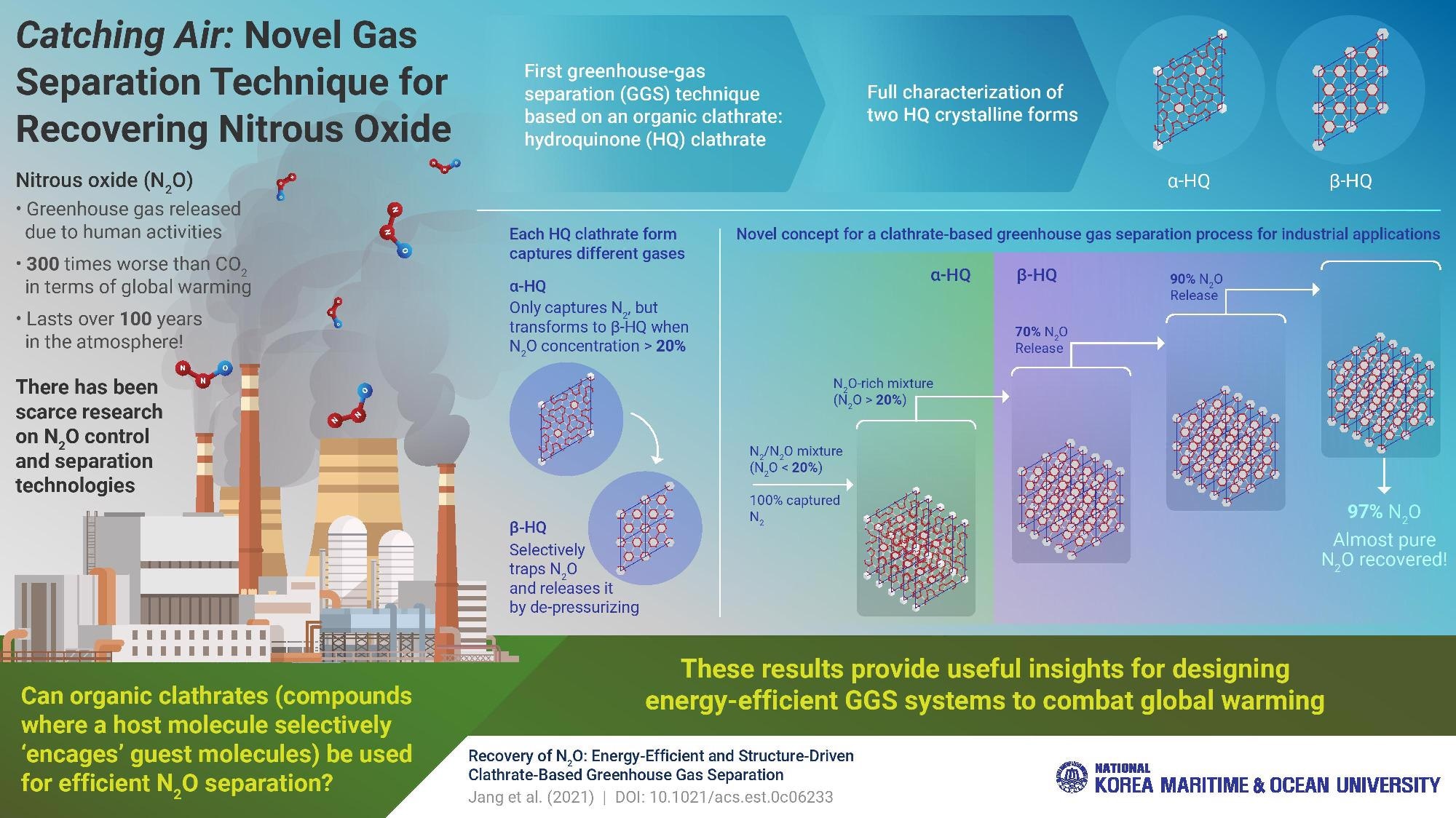
Image Credit: National Korea Maritime & Ocean University
When speaking of global warming and greenhouse gases, people immediately think of carbon dioxide (CO2) as the main villain. However, the truth is that there are other gases produced by human activities that are much worse. Such is the case for nitrous oxide (N2O), which has received less attention in spite of its 300-fold greater impact on global warming than CO2. “Gas separation technology focusing on CO2 has been the subject of much study, whereas the corresponding research on N2O recovery remains insufficient,” remarks Prof. Jiho Yoon from Korea Maritime and Ocean University.
Prof. Yoon’s research group has been focused on finding a feasible and energy-effective approach to separate and capture N2O from gas mixtures found in today’s industries. In their latest study, which was published in Environmental Science and Technology, they presented a new concept for greenhouse gas separation using hydroquinone (HQ) clathrate. Clathrates are a type of crystalline compound in which small molecules called guests are trapped inside appropriately sized holes or cages formed by host molecules.
Using a wide variety of techniques, including X-ray diffraction, scanning electron microscopy, Raman spectroscopy, and nuclear magnetic resonance, the scientists fully characterized two of the main crystalline forms of HQ, called α-HQ and β-HQ. They also analyzed the gas storage capacity and formation kinetics of both types, shedding light on the structure-driven selectivity of each form towards different gases.
It turns out that HQ clathrate behaves markedly differently depending on the concentration of N2O when subjected to a N2O/N2 mixture. For an N2O concentration below 20%, α-HQ traps only nitrogen (N2) molecules, acting like a highly selective molecular sieve. Conversely, when exposed to higher concentrations of N2O, α-HQ transforms into β-HQ, which primarily encages N2O molecules. Inspired by this newly found dual-function—or “Janus”— behavior of HQ clathrate, the scientists developed a novel concept for greenhouse gas separation. Their stepwise process involves using β-HQ across multiple chambers to sequentially obtain a purer and purer N2O, reaching a final concentration of about 97%. Meanwhile, α-HQ can be used to remove N2 from gas mixtures to help reach the initially required N2O concentration above 20%.
This study marks the first time an organic clathrate was used for N2O separation. Prof. Yoon highlights that their novel approach is applicable at room temperature and atmospheric pressure, which would make its adoption more feasible in the industry. “Our approach to N2O separation via clathrate formation may offer the dual benefits of greenhouse gas control and considerable energy savings, making it a promising option in terms of energy consumption and capital cost,” he concludes.
Let us hope that this technology is further developed, in order to secure a better future for our planet.