Jun 10 2021
Extensive adoption of hydrogen-powered vehicles over conventional electric vehicles needs fuel cells that can safely convert oxygen and hydrogen into water — a major implementation issue.
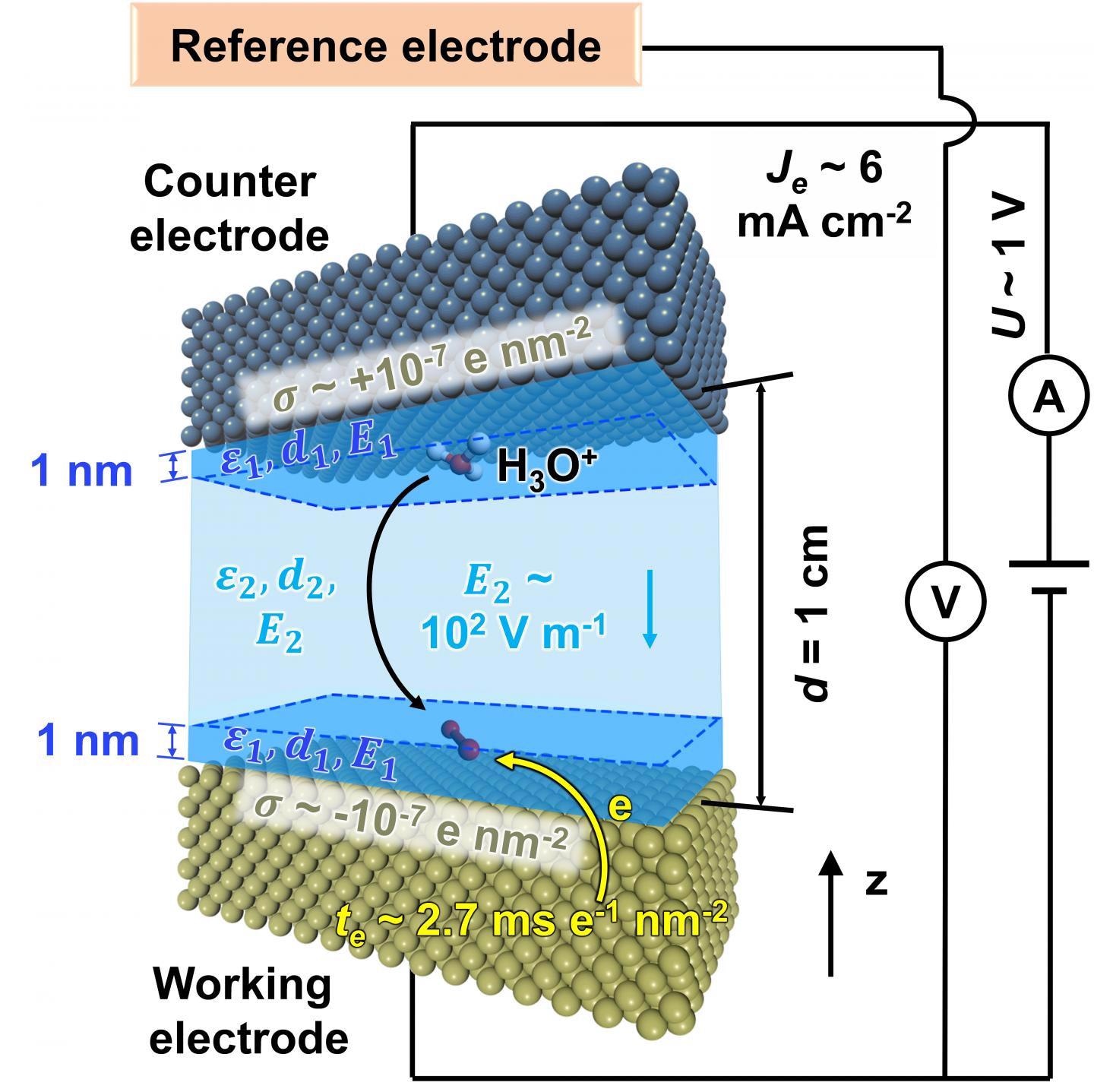 Schematic inner workings of the electrodes in a fuel cell, and the importance of key parameters. Image Credit: Heinz et al., 2021.
Schematic inner workings of the electrodes in a fuel cell, and the importance of key parameters. Image Credit: Heinz et al., 2021.
Scientists from the University of Colorado Boulder are now dealing with one aspect of that barrier by designing new computational models and tools required to better understand and control the conversion process.
Hendrik Heinz, an associate professor from the Department of Chemical and Biological Engineering, is heading the study in collaboration with the University of California Los Angeles. Recently, Heinz’s team has published the latest results on this topic in the journal, Science Advances.
Fuel cell electric vehicles integrate hydrogen in a tank along with atmospheric oxygen to generate the electricity required to run the vehicle. These vehicles do not have to be plugged in to charge, and they also provide an additional benefit of generating water vapor as a byproduct. These features, including other factors, have made them a fascinating option in the green and renewable energy transportation fields.
Heinz said that the main goal in making these vehicles feasible is to identify an efficient catalyst in the fuel cell that can “burn” hydrogen with oxygen under regulated conditions required for safe travel.
Scientists are also searching for a catalyst that can perform this task at near room temperatures, with an extended lifetime and high efficiency in an acidic solution. Platinum metal is generally utilized for this purpose, but anticipating the reactions and the most optimal materials to use for scaling up or for varied conditions has been a challenge so far.
For decades, researchers have struggled to predict the complex processes needed for this work, though enormous progress has been made using nanoplates, nanowires and many other nanostructures.
Hendrik Heinz, Associate Professor, Department of Chemical and Biological Engineering, University of Colorado at Boulder
“To address this, we have developed models for metal nanostructures and oxygen, water and metal interactions that exceed the accuracy of current quantum methods by more than 10 times. The models also enable the inclusion of the solvent and dynamics and reveal quantitative correlations between oxygen accessibility to the surface and catalytic activity in the oxygen reduction reaction,” added Heinz.
According to Heinz, the quantitative simulations developed by his team demonstrate the interaction between oxygen molecules as they come across different obstacles posed by molecular layers of water on the surface of platinum. Such interactions make the difference between a fast or slow follow-on reaction and should be regulated for the process to work efficiently.
Such reactions take place very quickly — it takes around a millisecond per square nanometer to complete the conversion into water — and occur on the surface of a tiny catalyst. All these variables combine together in a complex, intricate “dance” that was discovered by Heinz’s team to model in predictive ways.
Heinz added that the data-intensive and computational techniques explained in the article can be used to make designer-nanostructures that would boost the catalytic efficiency and also enable potential surface modifications to further improve the cost-benefit ratio of fuel cells.
Heinz’s collaborators are investigating the commercial significance of that aspect and Heinz is employing the tools to explore a broader range of promising alloys and gain a deeper understanding of the mechanics at play.
The tools described in the paper, especially the interface force field for order-of-magnitude more reliable molecular dynamics simulations, can also be applied to other catalyst and electrocatalyst interfaces for similar groundbreaking and practically useful advances.
Hendrik Heinz, Associate Professor, Department of Chemical and Biological Engineering, University of Colorado at Boulder
Journal Reference:
Wang, S., et al. (2021) Direct correlation of oxygen adsorption on platinum-electrolyte interfaces with the activity in the oxygen reduction reaction. Science Advances. doi.org/10.1126/sciadv.abb1435.