Hydrogels exhibit an astonishing potential to swell and consume water. On a daily basis, they are utilized in nappies, dressings and more to lock moisture away.
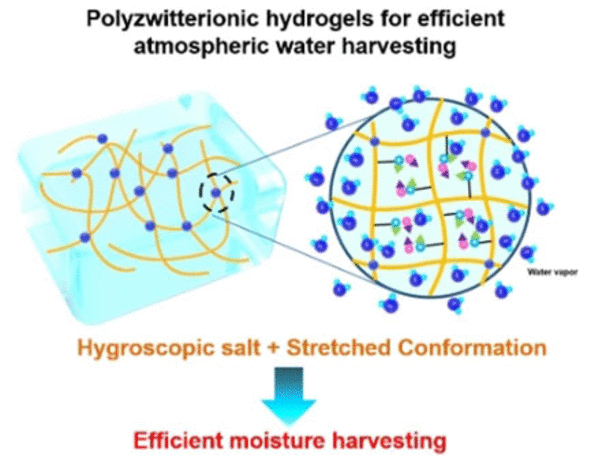
Image Credit: Angewandte Chemie
At present, a research group has discovered another use: rapidly extracting huge amounts of freshwater from the air with the help of a specially developed hydrogel consisting of a hygroscopic salt. The study reported in the journal Angewandte Chemie displays that the salt improves the moisture uptake of the gel, thereby making it ideal for water harvesting in dry regions.
Hydrogels have the ability to absorb and store many times their weight in water. By doing that, the underlying polymer swells significantly by incorporating water. But, so far, the use of this property to generate freshwater from atmospheric water has not been possible, as collecting moisture from the air is still extremely slow and inefficient.
At the same time, moisture absorption can be improved by adding hygroscopic salts that can quickly remove huge amounts of moisture from the air. But, hygroscopic salts and hydrogels are generally not compatible, as a large amount of salt impacts the swelling ability of the hydrogel and thereby degrades its properties. The salt ions are also not tightly coordinated inside the gel and are washed away easily.
Materials scientist Guihua Yu and his team at the University of Texas at Austin, USA, have overcome these problems by developing a “salt-friendly” hydrogel. As their study reveals, this gel has the potential to absorb and maintain water when integrated with a hygroscopic salt. With the help of their hydrogel, the team was able to extract nearly six liters of pure water per kilo of material in just 24 hours, from air having 30% relative humidity.
The basis for the new hydrogel was a polymer built from zwitterionic molecules. Polyzwitterions carry both positive and negative charged functional groups. In this case, this helped the polymer become more responsive to the salt.
The molecular strands in the polymer were initially tightly intermingled, but when the scientists added the lithium chloride salt, the strands relaxed and a porous, spongy hydrogel was developed. This hydrogel filled with hygroscopic salt was able to integrate water molecules in a quick and simple manner.
As a matter of fact, water incorporation was so rapid and simple that the team was able to fix a cyclical system for constant water separation. The hydrogel was left for an hour each time to absorb atmospheric moisture and then dried in a condenser to gather the condensed water. This procedure was repeated multiple times without it leading to any considerable loss of the amount of water that has been condensed, absorbed or collected.
Yu and the group state that the as-prepared hydrogel “should be optimal for efficient moisture harvesting for the potential daily water yield.” They conclude that polyzwitterionic hydrogels could play a major role in the coming years for recovering atmospheric water in drought-stricken, arid regions.
Journal Reference:
Lei, C., et al. (2022) Polyzwitterionic Hydrogels for Efficient Atmospheric Water Harvesting. Angewandte Chemie International Edition. doi.org/10.1002/anie.202200271