Reviewed by Alex SmithJun 1 2022
With the help of electrical current derived from renewable energy sources, green hydrogen at present has generally been created by splitting water. Researchers at Technische Universität Wien (TU Wien) have, however, come up with a new photocatalyst design that could make this process highly direct and controllable.
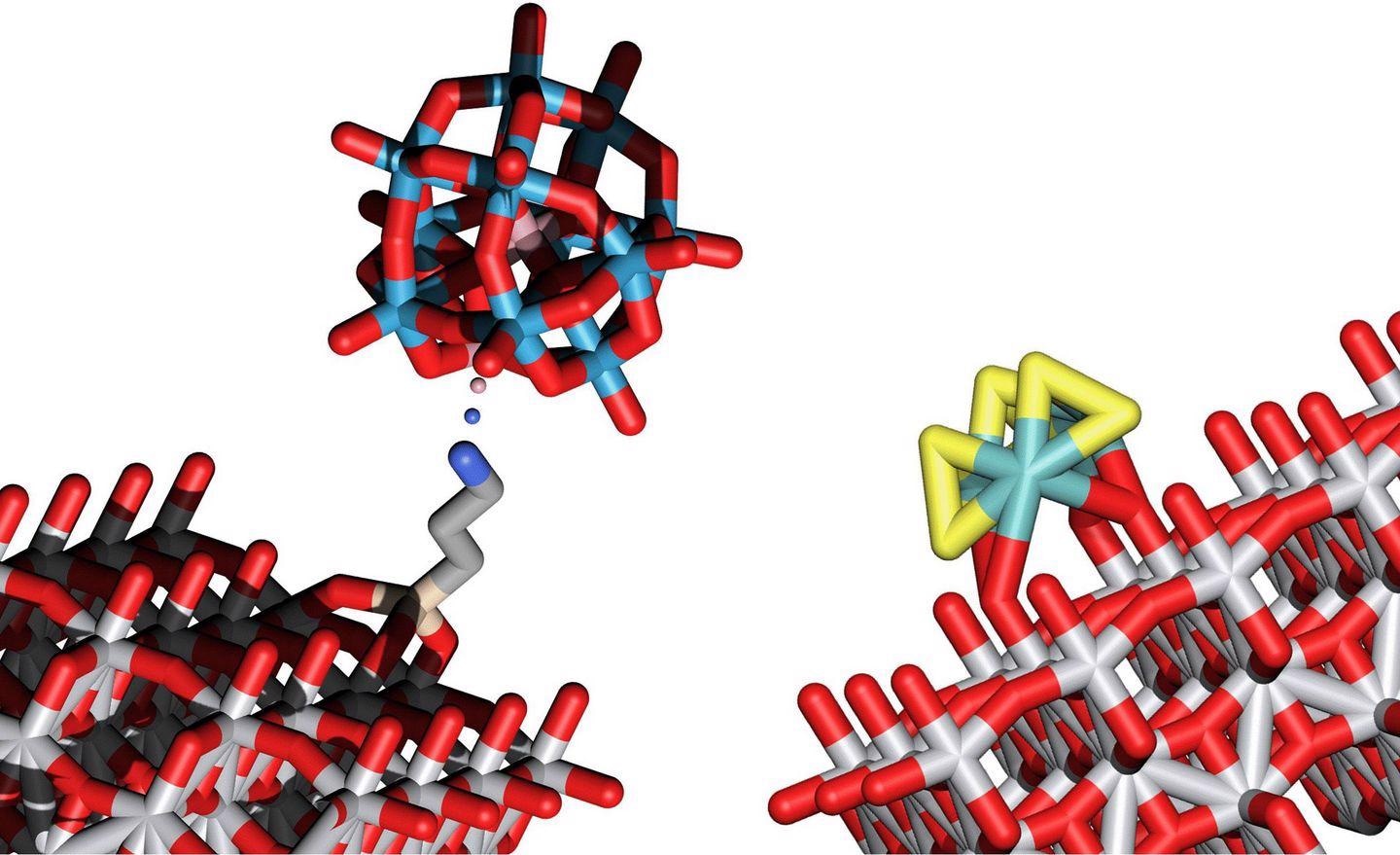 Structural models of two clusters that enable water splitting into O2 and H2 by means of light energy. Image Credit: Technische Universität Wien.
Structural models of two clusters that enable water splitting into O2 and H2 by means of light energy. Image Credit: Technische Universität Wien.
Hydrogen could be a significant part of the future energy supply: It could be stored, transported, and burned as required. But the majority of the hydrogen available at present is a by-product of natural gas production—this has to be modified for climate protection.
Until now, the best plan to produce eco-friendly so-called “green hydrogen” is by splitting water into oxygen and hydrogen utilizing electricity that is available from renewable energy sources, for instance, photovoltaic cells.
But it would be even easier if sunlight could be utilized directly to split water. This is really what new catalysts are making possible at present, in a process known as “photocatalytic water splitting”. The concept is not yet industrially utilized.
At TU Wien, significant steps have been taken in this direction: on an atomic scale, researchers identified a new combination of molecular and solid-state catalysts that could do the job based only on comparatively affordable materials.
Interaction of Different Atoms
Actually, to be able to split water with light you have to solve two tasks at the same time. We have to think about oxygen and about hydrogen. The oxygen atoms of the water must be transformed into O2 molecules, and the remaining hydrogen ions – which are just protons – must be turned into H2 molecules.
Alexey Cherevan, Institute for Materials Chemistry, Technische Universität Wien
Alexey Cherevan works in Professor Dominik Eder’s Research Group,
Currently, solutions have been discovered for both tasks: Small inorganic clusters comprising just a small number of atoms that are anchored on a surface of light-absorbing support structures like titanium oxide. The combination of clusters and cautiously selected semiconductor supports results in the preferred behavior.
The clusters accountable for oxidizing oxygen are comprised of tungsten, oxygen, and cobalt, while clusters of molybdenum and sulfur are particularly suitable for making hydrogen molecules. The scientists at TU Wien were the first to deposit such clusters on a surface made of titanium oxide, where they can serve as catalysts for water splitting.
Titanium oxide is sensitive to light, that was already well known. The energy of the absorbed light leads to the creation of free-moving electrons and free-moving positive charges in the titanium oxide. These charges then allow the clusters of atoms sitting on this surface to facilitate the splitting of water into oxygen and hydrogen.
Alexey Cherevan, Institute for Materials Chemistry, Technische Universität Wien
Precise Control, Atom by Atom
Alexey Cherevan stated, “Other research groups working on splitting water with light rely on nanoparticles that can take on very different shapes and surface properties. The sizes are hard to control, the atoms are not quite arranged in the same way. Therefore, in this case, it is not possible to explain exactly how the catalysis process takes place in detail.”
Simultaneously, at TU Wien, the proper structure of the clusters has been identified with atomic precision, enabling one to gain a complete understanding of the catalytic cycle.
This is the only way to get feedback on what the efficiency of the process really depends on. We don't want to just rely on a trial and error approach and try different nanoparticles until we find the best one - we want to find out at the atomic level what the optimal catalyst really is.
Alexey Cherevan, Institute for Materials Chemistry, Technische Universität Wien
As it has already been proven that the chosen materials are ideal for splitting water, the next step is to tune their precise structure to obtain even greater efficiencies.
Simple and Promising
Alexey Cherevan emphasizes, “The decisive advantage of our method over splitting water by electrolysis is its simplicity.
Initially, electric hydrogen production needs a supportable energy source like photovoltaic cells, such as an electric energy storage device, and an electrolysis cell.
All things considered; this leads to a comparatively complicated system comprising a multitude of raw materials. At the same time, for photocatalytic water splitting, all that is required is a suitably coated surface that has been covered by water and then irradiated by the sun.
In the long run, this understanding could also be utilized to produce highly complex molecules with the help of the idea of artificial photosynthesis. Also, it may even be possible to use the energy of solar radiation to generate hydrocarbons with carbon dioxide from the water and air. This can additionally be utilized for other applications.
Journal Reference:
Batool, S., et al. (2022) Surface Anchoring and Active Sites of [Mo3S13]2– Clusters as Co-Catalysts for Photocatalytic Hydrogen Evolution. ACS Catalysis. doi.org/10.1021/acscatal.2c00972.