As the cost of lithium-ion batteries continues to drop, they are rapidly replacing the lead-acid batteries that were previously used in cars and other vehicles. This is causing an abundance of used lead acid batteries, which are hazardous to the environment and people if not properly recycled.
 Researchers developed an environmentally friendly method to turn lead from used lead acid batteries into photodetectors operating in the UV-visible band. They created lead(II)iodide (PbI2) microcrystals from the lead paste found in batteries using a one-pot solution process. Image Credit: Longxing Su, Southern University of Science and Technology.
Researchers developed an environmentally friendly method to turn lead from used lead acid batteries into photodetectors operating in the UV-visible band. They created lead(II)iodide (PbI2) microcrystals from the lead paste found in batteries using a one-pot solution process. Image Credit: Longxing Su, Southern University of Science and Technology.
To address this issue, researchers devised an environmentally friendly method for converting lead from used lead acid batteries into photodetectors operating in the UV-visible band.
We believe this recycling strategy could significantly reduce the lead pollution resulting from waste lead acid batteries, which is important to the environment. The photodetectors promote the recycling economy by creating a market for recycled lead. They can be used for a variety of applications including optical communication, chemical analysis, and imaging.
Longxing Su, Research Team Leader, Southern University of Science and Technology
Su and co-workers elucidate their process for retrieving lead from discarded lead acid batteries and then using it to produce lead(II)iodide (PbI2) microcrystals appropriate for use in photodetectors in the Optica Publishing Group journal Optics Letters.
The recycled PbI2 microcrystals exhibit the quality and purity levels necessary for making photodetectors. We also show that the microcrystals can be used to make photodetectors with excellent stability, repeatability, and fast response speeds.
Longxing Su, Research Team Leader, Southern University of Science and Technology
A New Use for Old Batteries
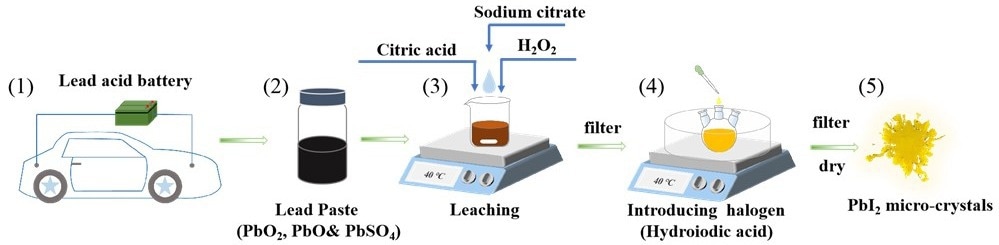
Even though the lead present in used lead acid batteries can be recycled, most techniques are expensive and have a number of drawbacks. Su’s team devised a more efficient method for producing PbI2 from lead paste found in lead acid batteries.
The researchers developed a one-pot process to extract the lead from the paste that uses only cost-efficient, readily accessible chemicals and no commercial precursors, which would increase the cost. The paste is placed in a solution containing excess citric acid monohydrate, sodium citrate dihydrate, and H2O2.
Because of the excess sodium citrate dihydrate, almost all of the generated lead citrate dissolves in the mixed solution. The mixture is then filtered to obtain a clear lead-containing solution. When excess hydroiodic acid is added to the solution, a yellow PbI2 precipitate is formed, which is retrieved and dried in a vacuum.
The PbI2 produced by this process was then used to create a photodetector using a simple spin-coating process. They investigated the photodetector’s photo response using a UV-Visible light source of 300 W Xe-lamp and an electric signal collector of a semiconductor parameter instrument. Under 10 V bias voltage, the PbI2 photodetector they built had a low dark current of 1.06 nA and an on–off ratio of 103.
Next Steps
The investigators are now attempting to scale up their process to mass-produce recycled PbI2. Prior to commercialization, independent testing organizations and companies interested in incorporating the photodetectors into downstream products would need to verify the recycled PbI2 and photodetectors made from it.
We hope that our work will be noticed by chemical companies and downstream firms so that our method can be extended into the market. Our green reactant recycling method may also be useful for other applications, such as making solar cells.
Longxing Su, Research Team Leader, Southern University of Science and Technology
Journal Reference:
Su, L., et al. (2023) A photodetector fabricated from 2H-PbI2 micro-crystals recycled from waste lead acid batteries. Optics Letters. doi.org/10.1364/OL.480972.