Since the beginning of the Industrial age, the concentration of carbon dioxide (CO2) in the Earth’s atmosphere has increased year-on-year and continues to do so. This has led scores of researchers to investigate methods for extracting CO2 from the air.
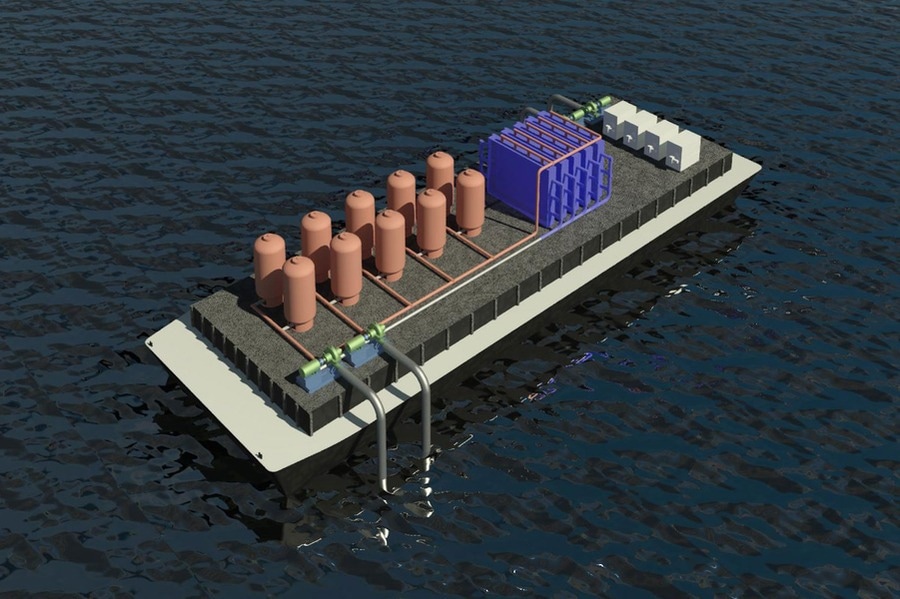
Image Credit: Courtesy of the researchers from MIT
Recently, a team of researchers at MIT has turned its attention to the ocean, which acts as a CO2 reservoir, absorbing 30 to 40 percent of the atmospheric gas produced by human activity.1
Published in the journal Energy and Environmental Science, the MIT team report that they have discovered an innovative and effective method for removing CO2 from ocean water which could be carried out by ships as they navigate the ocean waters. The system could also be fitted to offshore drilling platforms or fish farms, expanding the scope of the system.
Net Negative Potential
The possibility of effectively removing CO2 from the oceans further boosts the potential of mitigating CO2 discharge while perhaps moving towards the goal of carbon neutral or net negative emissions.
The current method used to remove CO2 from ocean water is water splitting, which applies a voltage across a series of stacked bipolar membranes. This method breaks the water down by converting bicarbonates in the water into molecules of CO2, which can then be extracted via a vacuum. However, water splitting typically requires expensive materials and chemicals to perform the task.
We wanted to avoid the need for introducing chemicals to the anode and cathode half cells and to avoid the use of membranes if at all possible.
T. Alan Hatton, the Ralph Landau Professor of Chemical Engineering, MIT
The MIT team was able to create a membrane-free process that uses reactive electrodes rather than expensive bipolar membranes in a cyclic process in order to extract CO2 from the ocean.
The Process
The innovative method works by acidifying the water, which begins the conversion process of the dissolved bicarbonates to produce CO2 molecules using the reactive electrodes.
These molecules are then extracted using a vacuum as the water is pumped to a set of cells with the voltage reverse, where the water is converted back to an alkaline state so it can be safely released into the ocean.
What this process achieves is the reversal of the acidification of the oceans, which is a result of CO2 buildup.
Acidic oceans are responsible for the bleaching of coral reefs and threaten many marine species, including shellfish. The idea is that by rolling out this system at scale, including on ships traversing the seas, the method could help alleviate the impact of CO2 caused by human activity.
We’re not going to be able to treat the entire planet’s emissions … This could help shipping companies offset some of their emissions, and turn ships into ocean scrubbers.
Professor Kripa Varanasi, MIT
Once the extraction of CO2 has been achieved, there is still the need to dispose of or store this greenhouse gas without causing any release into the atmosphere.
You can certainly consider using the captured CO2 as a feedstock for chemicals or materials production, but you’re not going to be able to use all of it as a feedstock… You’ll run out of markets for all the products you produce, so no matter what, a significant amount of the captured CO2 will need to be buried underground.
T. Alan Hatton, the Ralph Landau Professor of Chemical Engineering, MIT
Moreover, as the concentration of CO2 in the oceans is over 100x greater than that of the air, the system could prove to be more significant and effective than current air-capture methods as only the extraction of the gas is required with the capture step already completed as the ocean absorbs CO2 directly.
Carbon dioxide is one of the most significant challenges we face when it comes to achieving climate goals and mitigating the effects of the climate crisis. With this in mind, the team at MIT anticipates that the system will be ready within the next two years for demonstration purposes, hopefully aiding in the fight against climate change.
References and Further Reading
- Chandler, D.L. (2023) How to pull carbon dioxide out of seawater, MIT News | Massachusetts Institute of Technology. Available at: https://news.mit.edu/2023/carbon-dioxide-out-seawater-ocean-decorbonization-0216
- Kim, S. et al. (2023) “Asymmetric chloride-mediated electrochemical process for CO2 Removal from Oceanwater,” Energy & Environmental Science. Available at: https://pubs.rsc.org/en/Content/ArticleLanding/2023/EE/D2EE03804H
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.