The Tokyo University of Agriculture and Technology (TUAT) research team created and developed a unique recycling process for generating biomass-derived chemicals by reusing the target product’s byproducts. Using numerical simulations, the research team developed a self-sustaining mechanism that minimizes the use of external utilities in the chemical process in this study.
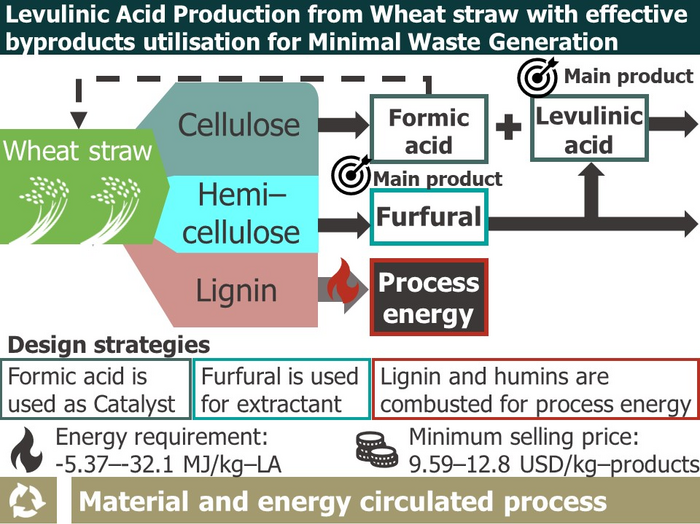 The conceptual diagram of this study. Image Credit: Ryu Ukawa-Sato, Tokyo University of Agriculture and Technology
The conceptual diagram of this study. Image Credit: Ryu Ukawa-Sato, Tokyo University of Agriculture and Technology
This accomplishment has considerably contributed to energy conservation by utilizing by-products from the traditional process in a chemical process to assure profitability. While prior procedures were planned on a vast scale, this study was designed with a viable biomass supply in all regions. This is an entirely different approach than traditional techniques.
With this success, researchers can considerably reduce future petroleum usage in chemical processes, paving the way for a recycling-oriented society. A chemical industry for local manufacture and consumption using agricultural and forestry waste in mountainous regions is projected to be established for regional revitalization.
The findings were published in Chemical Engineering Research and Design.
Biomass is a renewable carbon resource that can replace fossil fuels in chemical manufacturing. Among the biomass-derived chemicals, levulinic acid (LA) is gaining popularity as a platform chemical since it can be synthesized from cellulose, which accounts for approximately 50% of woody biomass, and is a precursor for a wide range of substances ranging from pharmaceuticals to biofuels.
Conventional biomass-based LA production systems are large-scale, with an annual biomass supply of more than 120,000 tons, and have faced difficulty in effectively utilizing all biomass resources.
As a result, the research team has created a technique that lowers the supply of external utilities by reusing as many by-products produced during the synthesis of LA from biomass as feasible while still supplying all of the required energy from the combustion of by-products.
Notably, the entire process energy was lowered and supplied by itself by recycling the by-product formic acid as a catalyst, using a chemical called furfural generated from biomass as an extraction solvent to purify LA, and combusting the solid by-products. It was also discovered that the extra solid by-products might be successfully used for building materials and other purposes.
Researchers also discovered that using river water might supply the necessary cooling. The amount of river water in the middle reaches of the Naka River in Tochigi Prefecture, Japan’s rural area, is less than 0.12% of the minimum annual volume flow.
In terms of economics, the minimum selling price of this process’s LA was $9.59 per kg, which was higher than the market price of $7.17 per kg. The rationale is that this technique allows for thin profit margins, an advantage of large-scale processes, and no separation and purification of by-products.
According to the above points, the findings of this study indicate that this procedure can be implemented entirely in society for a locally produced and consumed chemical industry in mountainous regions employing agricultural and forestry waste.
This research was funded by the Tokyo University of Agriculture and Technology’s Doctoral Program for World-leading Innovative and Smart Education (WISE Program of TUAT): “Excellent Leader Development for Super Smart Society by New Industry Creation and Diversity” granted by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Journal Reference
Ukawa-Sato, R., et al. (2023). Design and techno–economic analysis of levulinic acid production process from biomass by using co-product formic acid as a catalyst with minimal waste generation. Chemical Engineering Research and Design. doi.org/10.1016/j.cherd.2023.02.046.