As part of efforts to develop a decarbonized economy, green hydrogen, which produces hydrogen without the use of fossil fuels or the emission of carbon dioxide, has gained popularity recently.
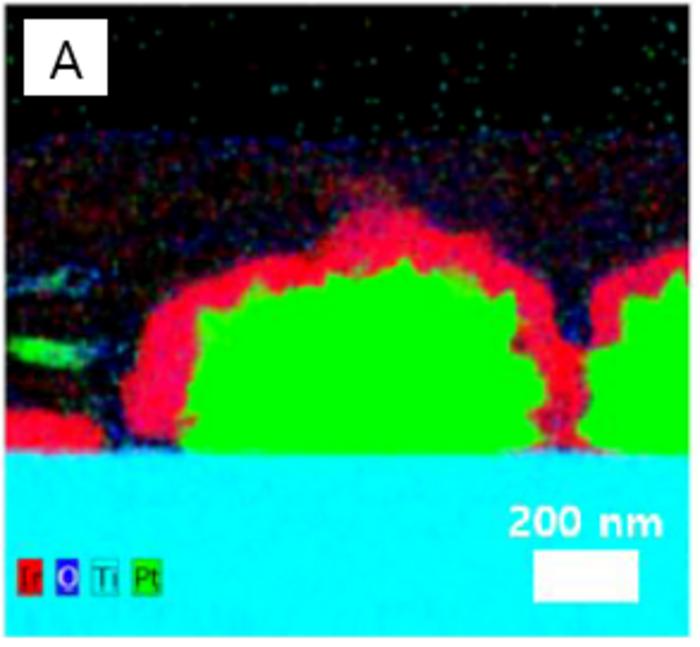 (A) Catalyst shapes made with conventional technology (red-iridium catalyst/green-platinum). Image Credit: Korea Institute of Science and Technology.
(A) Catalyst shapes made with conventional technology (red-iridium catalyst/green-platinum). Image Credit: Korea Institute of Science and Technology.
However, the economic viability of green hydrogen has not been very high due to the high manufacturing cost of water electrolysis devices that generate green hydrogen. However, the discovery of a technique that substantially reduces the amount of rare metals utilized in polymer electrolyte membrane water electrolysis devices, like iridium and platinum, is paving the way for lower production costs.
Dr. Hyun S. Park and Sung Jong Yoo of the Korea Institute of Science and Technology’s (KIST) Hydrogen and Fuel Cell Research Center announced the development of a technology that can substantially decrease the quantity of platinum and iridium, precious metals employed in the electrode protection layer of polymer electrolyte membrane water electrolysis devices while maintaining performance and durability comparable to existing devices.
In particular, the researchers replaced the expensive precious metal in the electrode protection layer with inexpensive iron nitride with a greater surface area and homogeneously coated a small amount of iridium catalyst on top of it, greatly enhancing the economic effectiveness of the device. This is in contrast to prior studies that focused on reducing the amount of iridium catalyst while maintaining the structure that uses a large amount of platinum and gold as the electrode protection layer.
The polymer electrolyte membrane water electrolysis device creates high-purity hydrogen and oxygen by decomposing water using electricity supplied by renewable energy sources such as solar power, and it is used to provide hydrogen to industries like steelmaking and chemicals. Furthermore, because it is beneficial for energy conversion to store renewable energy as hydrogen energy, enhancing the economic efficiency of this device is critical for achieving a green hydrogen economy.
There are two electrodes in a typical electrolysis device that generates hydrogen and oxygen, and for the oxygen-generating electrode, which works in an extremely corrosive environment, gold or platinum is coated on the surface of the electrode at 1 mg/cm2 as a protective layer to guarantee durability and production efficiency, and 1–2 mg/cm2 of iridium catalyst is coated on top.
The precious metals utilized in these electrolysis devices have extremely limited deposits and production, which is a major impediment to the widespread adoption of green hydrogen generation devices.
To enhance the economics of water electrolysis, the researchers employed iron nitride (Fe2N) instead of the precious metals gold and platinum as a protective layer for the oxygen electrode in polymer electrolyte membrane hydrogen generation devices.
To do this, the researchers devised a composite process that coats the electrode uniformly with iron oxide, which has poor electrical conductivity and then transforms the iron oxide to iron nitride, which has higher conductivity.
The team also developed a procedure for uniformly coating an iridium catalyst around 25 nanometers (nm) thick on top of the iron nitride protective layer, resulting in an electrode with high hydrogen production efficiency and durability.
The designed electrode substitutes the gold or platinum used as a protective layer for the oxygen-generating electrode with non-precious metal nitrides while retaining comparable performance to existing commercial electrolysis units and reducing the amount of iridium catalyst to 10% of the current level. Furthermore, the electrolysis unit with the new components was run for over 100 hours to ensure its initial stability.
Reducing the amount of iridium catalyst and developing alternative materials for the platinum protective layer are essential for the economical and widespread use of polymer electrolyte membrane green hydrogen production devices, and the use of inexpensive iron nitride instead of platinum is of great significance. After further observing the performance and durability of the electrode, we will apply it to commercial devices in the near future.
Dr. Hyun S. Park, Korea Institute of Science and Technology
The study was funded by the Ministry of Trade, Industry and Energy (Minister Lee, Chang-Yang) and KIST Major Projects.
Journal Reference:
Jeong, H.-Y., et al. (2023). High–performance water electrolyzer with minimum platinum group metal usage: Iron nitride–iridium oxide core–shell nanostructures for stable and efficient oxygen evolution reaction. Applied Catalysis B: Environmental. doi.org/10.1016/j.apcatb.2023.122596.