There are multiple methods for harnessing solar energy to separate water and generate hydrogen. Regrettably, this eco-friendly hydrogen has remained pricier than natural gas-derived “grey” hydrogen.
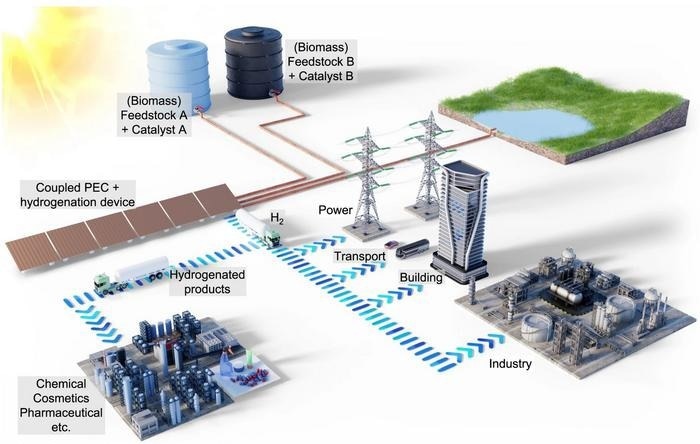
Illustration of the solar-powered coupled photoelectrochemical and hydrogenation unit. The coupled device uses sunlight to generate hydrogen and consumes some of the generated hydrogen in situ to hydrogenate biomass-derived feedstock using a homogeneous catalyst. The remaining hydrogen can then be used for energy storage, transport, building, and industrial applications. The hydrogenated products are valuable chemicals in high demand by the chemical and pharmaceutical industries. The fraction of the hydrogen used for the hydrogenation reaction can be adapted to demand by regulating the supply of biomass feedstock and the catalyst concentrations accordingly. By switching between "Feedstock A + Catalyst A" and "Feedstock B + Catalyst B", different hydrogenation products can be produced in the same PEC plant. Image Credit: Hassan Tahini, ScienceBrush Design
A joint study by Helmholtz-Zentrum Berlin (HZB) and the Technical University of Berlin has recently demonstrated a pathway to making solar-derived green hydrogen economically viable. A portion of the hydrogen is utilized for enhancing the quality of raw biomass-derived chemicals, transforming them into high-value industrial chemicals. This co-production approach offers remarkable flexibility, enabling the same facility to generate various by-products based on demand.
To curb global warming, a swift transition away from fossil fuels is imperative. In the forthcoming energy landscape, green hydrogen will assume a pivotal role in energy storage and serve as a sustainable raw material for manufacturing various chemicals and materials across diverse applications.
Currently, hydrogen primarily originates from fossil natural gas, termed “grey” hydrogen. In contrast, “green” hydrogen is manufactured through water electrolysis, driven by renewable energy sources. A promising avenue involves the utilization of photoelectrochemical (PEC) devices to produce hydrogen via solar energy. Nonetheless, hydrogen derived from PEC facilities is considerably costlier compared to hydrogen obtained from fossil methane sources.
Full Control Over Reactions
A team, led by Fatwa Abdi (formerly at HZB until mid-2023, now at City University in Hong Kong) and Reinhard Schomäcker (UniSysCat, TU Berlin), has explored the shifting dynamics when a portion of the hydrogen generated in a photoelectrochemical (PEC) device engages in a chemical reaction with itaconic acid (IA), ultimately producing methylsuccinic acid (MSA), all within the same device.
The primary input, itaconic acid, is sourced from biomass and introduced into the system. Methylsuccinic acid holds significant value and is in demand within the chemical and pharmaceutical sectors. The study outlines the methodology for regulating these chemical reactions within the PEC device, achieved by adjusting process parameters and the concentration of the water-soluble rhodium-based catalyst, which remains active at room temperature.
This approach allows for the selective modulation of hydrogen utilization in the hydrogenation of itaconic acid, enabling the tailored adjustment of methylsuccinic acid production, either increasing or decreasing it as needed.
Plant Becomes Profitable From 11% Hydrogen for MSA
Considering a practical overall efficiency of 10% for a photoelectrochemical (PEC) plant and factoring in primary expenses as well as operational, maintenance, and decommissioning costs, the production of pure hydrogen remains economically less viable when compared to fossil gas production. This holds true even when assuming a 40-year operational lifetime for the PEC plant.
The economic equation undergoes a significant transformation when the PEC reaction is integrated with the hydrogenation process. Even with just 11% of the generated hydrogen being converted to MSA, the cost of hydrogen diminishes to 1.5 € per kilogram, matching the cost of hydrogen obtained through methane steam reforming.
Remarkably, this cost parity is maintained even with a relatively short PEC plant lifetime of just five years. Also, the market price of MSA, being substantially higher than that of hydrogen, further enhances profitability. In the experiments, a range from 11% to as much as 60% of the hydrogen could be judiciously directed toward MSA production.
Furthermore, a prior study has demonstrated that the co-production of MSA concurrently reduces the energy payback time, which refers to the duration required for the plant to recover the energy expended in its production processes.
Co-Production Can Be Switched
The versatility of the PEC plant extends to the production of various other co-products by merely altering the feedstock and the water-soluble catalyst. For instance, acetone could undergo hydrogenation to yield isopropanol.
This is another major advantage of our concept of co-production. We have found a promising way to make solar hydrogen production economically viable.
Fatwa Abdi, City University
The study was conducted within the framework of the Berlin Cluster of Excellence “UniSysCat” (Unifying Systems in Catalysis) and received backing from the Excellence Network initiative of the Helmholtz Association.
Journal Reference
Obata, K., et al. (2023). Solar-driven upgrading of biomass by coupled hydrogenation using in situ (photo)electrochemically generated H2. Nature Communications. doi.org/10.1038/s41467-023-41742-4.