Researchers in Japan have developed a new material for storing hydrogen energy efficiently at low cost to enable people to use environment-friendly energy sources instead of fossil fuels.
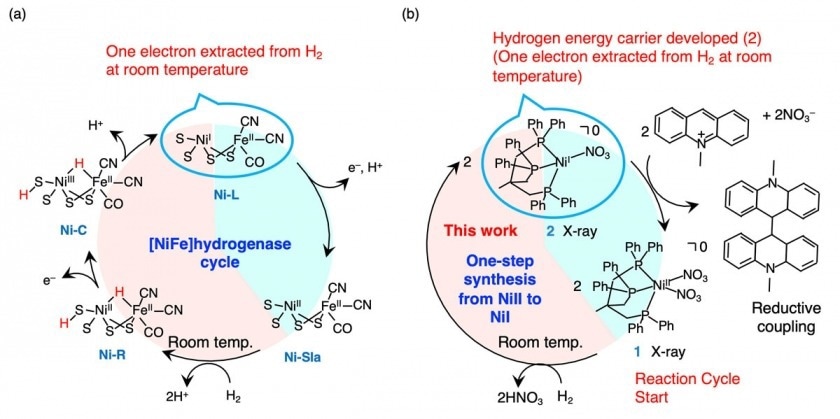 Comparing hydrogenases in nature with the compound developed in the current study. Comparison of the hydrogenase cycle found in nature with the compound developed in the study. Hydrogenases extract an electron from a single hydrogen molecule at room temperature for use in other biological processes. The compound developed in the current study undergoes a similar Image Credit: Kyushu University.
Comparing hydrogenases in nature with the compound developed in the current study. Comparison of the hydrogenase cycle found in nature with the compound developed in the study. Hydrogenases extract an electron from a single hydrogen molecule at room temperature for use in other biological processes. The compound developed in the current study undergoes a similar Image Credit: Kyushu University.
The new hydrogen energy carrier made of nickel can store the energy at a low cost for up to three months at room temperature. The study results were reported in Chemistry—A European Journal.
In the global effort to address the ongoing climate crisis, researchers are exploring various avenues, including the transition to alternative energy sources, such as hydrogen. Kyushu University has been at the forefront of this endeavor for several decades, dedicating efforts to finding more efficient methods for utilizing and storing hydrogen energy, with the ultimate goal of achieving a carbon-neutral society.
We have been working on developing new materials that can store and transport hydrogen energy. Transporting it in its gaseous state requires significant energy. An alternative way of storing and transporting it would be to 'split-up' the hydrogen atoms into its base components, electrons, and protons.
Seiji Ogo, Professor, International Institute for Carbon-Neutral Energy Research, Kyushu University
Several candidates have been considered possible hydrogen energy carriers, such as ammonia, formic acid, and metal hydrides, without deciding the final energy carrier.
So, we looked to nature for hints. There are a series of enzymes called hydrogenases that catalyze hydrogen into protons and electrons and can store that energy for later use, even at room temperature. By studying these enzymes our team was able to develop a new compound that does exactly that.
Seiji Ogo, Professor, International Institute for Carbon-Neutral Energy Research, Kyushu University
The researchers at Kyushu University have achieved a significant breakthrough in the field of hydrogen energy. Their newly developed compound not only has the ability to extract and store electrons at room temperature but also serves as its own catalyst for electron extraction, a capability previously unattainable with hydrogen energy carriers. Additionally, the team demonstrated that this energy can be stored for up to three months.
An important aspect of this development is that the compound utilizes a cost-effective element, nickel, which stands in contrast to previous catalysts that relied on expensive metals like platinum, rhodium, or iridium. With nickel as a viable option for hydrogen energy storage, there is potential for a reduction in the cost of future compounds.
The research team is now eager to collaborate with the industrial sector to translate their groundbreaking findings into practical applications.
We would also like to work on improving storage time and efficiency as well as investigate the viability of cheaper metals for such compounds. Hopefully our findings will contribute to the goal of decarbonization so that we can build a greener and environmentally friendly future.
Seiji Ogo, Professor, International Institute for Carbon-Neutral Energy Research, Kyushu University
Journal Reference:
Takahashi, C., et al. (2023). Single-Step Synthesis of Nil from Nill with H2. Chemistry—A European Journal. doi.org/10.1002/chem.202302297