To address climate change, the world needs to see a pressing shift towards sustainable energy and innovative methods to convert greenhouse gases, especially carbon dioxide, into valuable resources. As emissions continue to drive global temperature and sea level rises, along with more frequent extreme weather events, the detrimental effects on ecosystems, human health, and economies intensify. Thus, it is crucial to reduce our reliance on fossil fuels as our main source of energy.
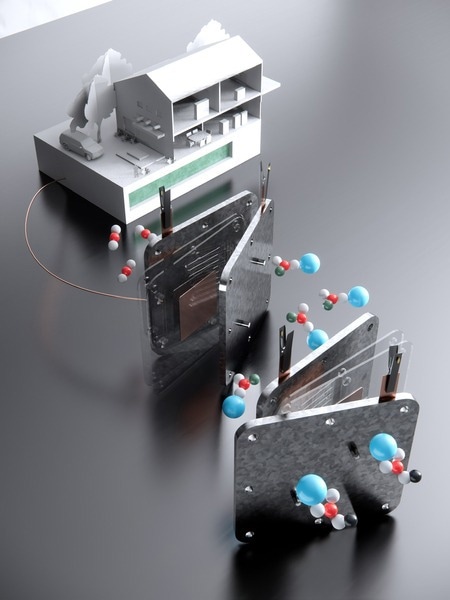
Image Credit: Shuhan Miao, Harvard Graduate School of Design
To this end, researchers from MIT and Harvard University have published the results of a groundbreaking research project that describes the development of an efficient process that can convert carbon dioxide into formate, a material that can be used in fuel cells to generate electricity. This groundbreaking innovation demonstrates how carbon dioxide can be used to develop fuel, opening the door to a solution that can simultaneously tackle the amount of greenhouse gases in the atmosphere while producing fuel in a more sustainable way.
Formate Fuel: The Sustainable Alternative
For some years, scientists have attempted to establish new methods of capturing carbon dioxide from the atmosphere to mitigate climate change. One of the more encouraging concepts that have emerged involves converting carbon dioxide into sustainable fuel that is able to substitute traditional fossil fuels.
Many of the techniques that have previously been developed to achieve this have, however, faced the challenges of low carbon efficiency and the production of hazardous fuels that are prone to combustion.
Scientists from MIT and Harvard University have developed an effective method of transforming carbon dioxide into formate, a material that exists in both solid and liquid states. Formate can be used similar to hydrogen or methanol to provide power to a fuel cell and produce electricity. The material is non-toxic, non-flammable, and easy to store and transport, making it ideal for use as a fuel.
The new method, spearheaded by MIT doctoral candidates Zhen Zhang, Zhichu Ren, and Alexander H. Quinn, was recently published in the journal Cell Reports Physical Sciences.
This approach captures carbon dioxide and electrochemically converts it into a solid formate powder, which can then be turned into electricity. While the initial demonstrations in the laboratory were on a smaller scale, the researchers are optimistic about scaling up the process. Future implementations might offer emission-free heating and electricity for households and have the potential for broader industrial or grid-scale applications.
The Breakthrough Conversion Process
Traditional techniques of converting carbon dioxide into fuel rely on a two-phase process. Initially, the gas undergoes a chemical process to be solidified. Subsequently, it is subjected to heating, both to release the trapped carbon dioxide and to convert it into a usable fuel. However, this latter phase proves inefficient, often only managing to convert a mere 20% of the carbon dioxide into the intended product.
In stark contrast, the innovative method introduced by the MIT team boasts an impressive conversion rate exceeding 90%. This process sidesteps the inefficient heating phase, opting instead to transform the carbon dioxide directly into liquid metal bicarbonate.
From there, it is transitioned into either liquid potassium or sodium formate using an electrolyzer powered by eco-friendly energy sources. The end product is a highly concentrated formate solution, which can be dried and stored as a durable solid powder, potentially for several years or even decades.
Practical Implications and Potential Applications
The fuel derived from this innovative method promises versatile applications. It holds the potential to energize anything from individual homes to expansive grid-scale storage systems. Initial applications could entail the creation of an electrolyzer unit, roughly the dimensions of a standard refrigerator, specifically tailored to capture and convert carbon dioxide into formate for domestic usage.
Any excess formate can then be stored in tanks either above ground, such as on rooftops, or below ground. In times of power need, this stored solid formate can be mixed with water and funneled into a fuel cell to provide homes with electricity.
This groundbreaking work showcases a proficient method for converting carbon dioxide into fuel. This approach is poised to play a crucial role in reducing carbon emissions and supplying an environmentally friendly energy alternative.
References and Further Reading
- David L. Chandler | MIT News (no date) Engineers develop an efficient process to make fuel from carbon dioxide, MIT News | Massachusetts Institute of Technology. Available at: https://news.mit.edu/2023/engineers-develop-efficient-fuel-process-carbon-dioxide-1030 (Accessed: 02 November 2023).
- Environmental and Energy Study Institute (EESI) (2021) Fossil fuels, EESI. Available at: https://www.eesi.org/topics/fossil-fuels/description (Accessed: 02 November 2023).
- Sullivan, I. et al. (2021) Coupling electrochemical CO2 conversion with CO2 Capture, Nature News. Available at: https://www.nature.com/articles/s41929-021-00699-7 (Accessed: 02 November 2023).
- Li, J. et al. (2023) A carbon-efficient bicarbonate electrolyzer [Preprint]. Available at: https://chemrxiv.org/engage/chemrxiv/article-details/6472121dbe16ad5c57017ecf.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.