Oct 21 2009
A small molecule designed to detect cyanide in water samples works quickly, is easy to use, and glows under ultraviolet or "black" light. Although the fluorescent molecule is not yet ready for market, its Indiana University Bloomington creators report in the Journal of the American Chemical Society (now online) that the tool is already able to sense cyanide below the toxicity threshold established by the World Health Organization.
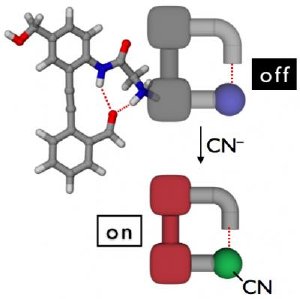 A ball-and-stick model of the detector is shown at upper left. After cyanide (CN-) reacts with the colorless detector, the detector will emit visible light when illuminated with ultraviolet or "black" light. Credit: Dongwhan Lee
A ball-and-stick model of the detector is shown at upper left. After cyanide (CN-) reacts with the colorless detector, the detector will emit visible light when illuminated with ultraviolet or "black" light. Credit: Dongwhan Lee
"This is the first system that works in water at normal pH levels and can be modified at will to enhance its reactivity," said IU Bloomington chemist Dongwhan Lee, who led the research. "We are now looking at how to make the detector more sensitive."
Graduate student Junyong Jo is the report's first author.
One of the reasons the detector is not ready for market, Lee says, is that its optical properties need to be improved to emit light at longer wavelengths with less interference from background signals, especially those of biological origin. Since pond or river water is likely to contain living organisms and other organic matter, Lee says the detector system must be perfected.
Another unique aspect of the detector molecule is its modular structure.
"This is an essentially three-component chemical device with an activator, a receptor, and a reporter module," Lee said. "These three components we can change at will in the future, either to make the detector more sensitive, or have it detect an entirely different toxin by sending out signals as different colors of light. Because of the structure's modularity, a change in one of the three components doesn't really affect the others."
Lee and Jo were inspired by life itself -- the natural properties of proteins -- when they began designing their sensor molecule. The design of this novel system takes advantage of the structure-organizing "beta turn" motif commonly found in protein structures. The detector is essentially inert, except in the presence of cyanide, with which it preferentially reacts. The addition of cyanide induces a subtle but important structural change in the detector that turns it into a pigment that absorbs ultraviolet light (currently 270 nm) and convert it to light emission at around 375 nm, a purplish color at the very edge of human beings' normal vision range.
Cyanide is a negatively charged ion composed of one carbon and one nitrogen atom. Among its many chemical targets inside cells is the oxidative phosphorylation system, which is a crucial producer of energy. Cyanide disrupts the system, making it impossible for cells to maintain even the most basic processes, which is one reason cyanide is considered a poison.
Cyanide is a common byproduct of industrial manufacturing.
In 2003 the World Health Organization reported that cyanide contamination of drinking water is a major problem in developing countries -- and in some developed countries, too. While cyanide contamination occasionally results in outbreaks of acute illness, in most cases, cyanide contamination levels are low enough that the health effects incurred in humans are less pronounced. In these cases, cyanide poisoning may simply present as anemia, goiter, or as a mysterious inability to maintain healthy vitamin B12 levels.
A fatal dose of cyanide in humans can be as little as 90 mg, or .003 ounces (in a body weighing 60 kg, or 132 pounds). U.S. Environmental Protection Agency guidelines suggest chronic minor health problems can result from a daily exposure to cyanide of .36 mg for a 60 kg body. Both the EPA and World Health Organization set the threshold of concern at around .72 mg per day for a 60 kg body.
Lee says he hopes to develop the detector system so that it can be used to protect people from inadvertently poisoning themselves with cyanide-laced drinking water.
"Considering that cyanide is a readily accessible chemical with fatal consequences, our approach to detect it under physiological conditions is a very exciting finding," Lee says.