Mar 10 2020
Researchers from King Abdullah University of Science and Technology (KAUST) have developed a simple, economical, and fast technique for modeling the combustion properties of gasoline. This latest development could result in transport fuels that are cleaner and more efficient
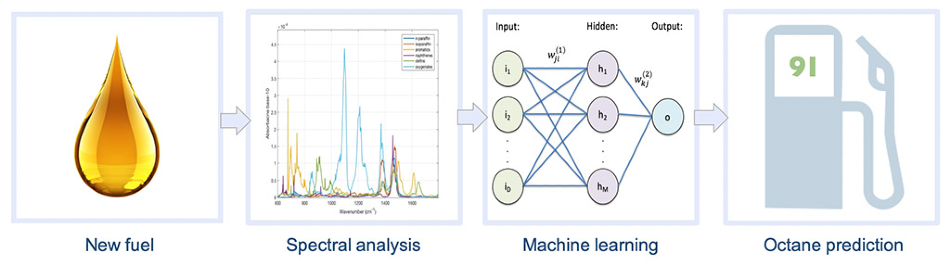 The workflow involves taking a new fuel to the infrared spectra and applying machine learning to perform an octane prediction. Image Credit: © 2020, KAUST.
The workflow involves taking a new fuel to the infrared spectra and applying machine learning to perform an octane prediction. Image Credit: © 2020, KAUST.
Hydrocarbon-based fuels are usually used for transportation, and this process considerably contributes to climate change. This has prompted the need for better-performing and cleaner fuels. The most frequently used fuel in cars is gasoline. This fuel contains large amounts of hydrocarbons and, based on its composition, has an extensive range of combustion properties.
Octane number is one of the indicators of a fuel’s performance—if the octane number is higher, more of the fuel is compressed at the time of ignition, and its combustion also becomes more efficient. However, it is costly, complicated, and time-intensive to physically quantify the octane rating for gasoline.
Aamir Farooq and Emad Al Ibrahim from Clean Combustion Research Center at KAUST have devised an easy and economical technique for modeling the gasoline’s combustion properties, which may help in detecting fuel mixtures that have high octane numbers.
Our model offers a quick and easy method for screening fuel mixture candidates without the need for physical testing. Researchers can use our model to theorize a new fuel blend and then estimate what its octane number would be.
Emad Al Ibrahim, Clean Combustion Research Center, KAUST
The scientists developed a data set containing octane numbers, infrared spectra, and molecular characteristics for the chief components of gasoline, such as aromatic hydrocarbons, paraffin, naphthene, olefin, and isoparaffin. Using this data set, they generated composite spectra for as many as 148 different blends of hydrocarbons.
With the help of a nonlinear statistical model, the researchers acquired the most appropriate data from the spectra. They subsequently transformed this information into scores relating to the fuel’s chemical properties. This method enabled the researchers to calculate the octane number of the fuel.
The use of nonlinear methods for analyzing spectra is important because hydrocarbon molecules tend to exhibit synergistic and antagonistic blending. For example, a mixture of two fuels can often produce an octane number that is higher than that of the individual constituents.
Emad Al Ibrahim, Clean Combustion Research Center, KAUST
The spectra for 38 FACE (“fuels for advanced combustion engines”) gasolines are simulated, and their octane numbers were precisely predicted using a nonlinear statistical model. This offered a way to determine the combustion properties of a wide range of fuel mixtures.
As we look to find newer and cleaner fuel formulations, we need to be able to quickly screen potential candidate fuels: low-carbon refinery blends, biofuels, solar fuels and e-fuels. We can now do this easily, cheaply and rapidly.
Aamir Farooq, Clean Combustion Research Center, KAUST
Fueling a cleaner future for transport
KAUST mechanical engineers have developed a simple and cost-effective method for modeling the combustion characteristics of gasoline, which could help to identify fuel mixtures with high octane numbers. Video Credit: © 2020, KAUST.