The Fukushima Daiichi nuclear catastrophe, set off by the earthquake and tsunami on March 11, 2011, caused a significant discharge of radioactive substances, notably cesium, from the compromised nuclear reactors.
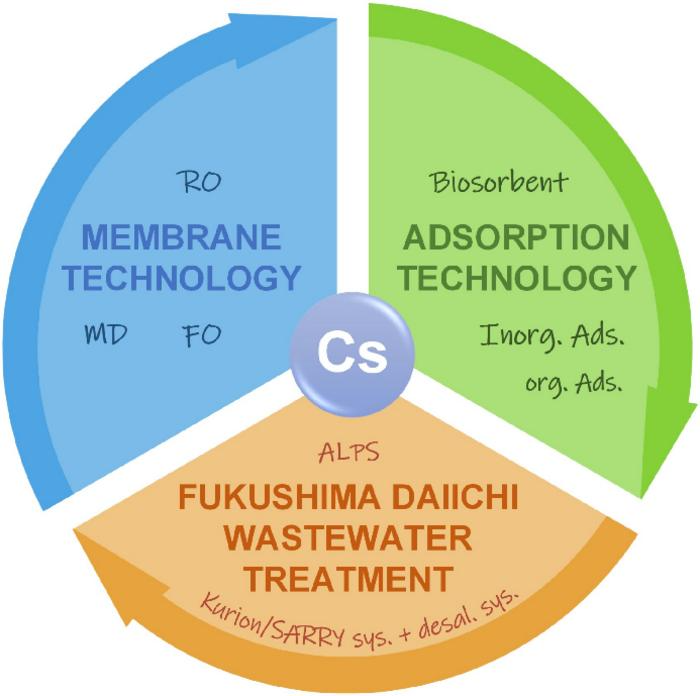
Image Credit: Higher Education Press Limited Company
The breakdown of cooling systems resulted in partial meltdowns of the reactor cores, releasing a considerable quantity of cesium-137 (Cs-137) and cesium-134 (Cs-134) into the surrounding environment.
Cs-137, in particular, presents significant environmental and human health risks due to its extended half-life and pronounced mobility in the environment. Environmentally, the release of Cs-137 contributes to radioactive contamination, potentially causing soil degradation and disruption of ecosystems.
From a human health perspective, prolonged exposure to Cs-137 radiation elevates the risk of cancer, particularly impacting the thyroid. The long-term consequences may involve chronic radiation damage, affecting immune and reproductive systems.
Extracting cesium from radioactive wastewater poses a formidable challenge attributed to various factors. The intricate chemical composition of cesium necessitates a technologically demanding removal process. Additionally, the considerable volume of radioactive wastewater generated, particularly in the aftermath of nuclear incidents like the Fukushima disaster, further complicates the situation.
Managing significant quantities of contaminated water requires large-scale treatment procedures, and selecting a suitable method must account for factors such as effectiveness, cost, and environmental repercussions.
Prof. Shuting Zhuang and Prof. Jianlong Wang's recent paper provides an in-depth examination of two prevalent techniques for cesium removal: adsorption and membrane separation methods.
Adsorption proves to be a highly efficient method for treating radioactive wastewater with low concentrations of radionuclides but encompassing a substantial volume. The primary emphasis is on advancing adsorbents that exhibit both high efficiency and cost-effectiveness for the removal of Cs+.
Diverse materials, including inorganic options such as hexacyanoferrates, carbon-based materials, clay minerals, geopolymers, MOFs (Metal-Organic Frameworks), and organic substances like resins, Cs+-imprinted polymers, macrocyclic ligands, as well as biological materials like microbes, industrial and agricultural wastes, and biopolymers, have been investigated for Cs+ removal.
Hexacyanoferrate, in particular, stands out for its exceptional adsorption capacity and selectivity in this context.
Membrane separation, specifically reverse osmosis (RO), emerges as a highly effective technique for cesium ion separation. RO membranes with smaller pores demonstrate efficient retention of cesium ions, providing advantages such as commercial viability, high efficiency, and favorable water fluxes.
However, it is worth noting that membrane methods result in concentrated retention liquid, requiring further treatment due to elevated concentrations of radioactive nuclides. Furthermore, the extended operation poses challenges related to the radiation stability of membrane materials.
Continued research and technological progress are crucial for the development of sustainable and cost-effective methods to treat contaminated water and address the environmental consequences of cesium discharge. Prof. Zhuang and Prof. Wang's article offers a comprehensive overview of recent advancements in this domain.
Journal Reference:
Zhuang, S., & Wang, J. (2023). Cesium removal from radioactive wastewater by adsorption and membrane technology. Frontiers of Environmental Science & Engineering. doi.org/10.1007/s11783-024-1798-1.