Mar 15 2016
A team of scientists at LiU’s Laboratory for Organic Electronics have produced an innovative supercapacitor that can be charged by solar energy. This patent-pending supercapacitor does not include any toxic or costly materials, and can be mass-produced on an industrial scale.
 Heat to electricity
Heat to electricity
With this latest breakthrough a new form of energy storage, charged by heat energy - for example when the sun shines during the day or when an industrial process releases waste heat - is possible in the near future. This heat is changed into electric current, which can then be stored until it is required. The study, headed by Professor Xavier Crispin, has been reported in the Energy Environmental Science journal.
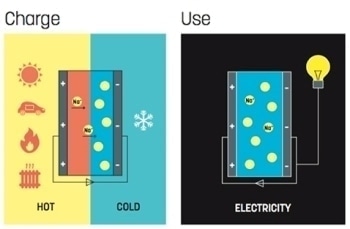
A supercapacitor is a type of battery that contains an electrolyte of ions, which are charged particles present between two electrodes. The charge obtained is usually stored in carbon nanotubes adjacent to the electrodes.
The research team used a physical phenomenon, where exposing a supercapacitor to a temperature gradient, i.e., keeping one end cold and the other end warm, causes the ions to travel towards the cold end, creating electricity. This thermoelectric effect is utilized to generate electricity of heat. The amount of heat which is changed into electricity relies on two factors - the type of electrolyte used and the extent of temperature variation.
For years, the team has worked with fluid electrolytes that contain conductive polymers and ions. Ions that are positively charged are tiny and fast, while polymer molecules that are negatively charged are large and bulky.
When one end cooled down and the other end is heated up the polymer chains remain where they are, while the tiny yet fast ions move towards the cold side. The ions easily bind to the metal electrodes. The charge that is produced is then stored in carbon nanotubes adjacent to the metal electrodes. When there is a need for electric current, this charge can be discharged.
After many years of research, Hui Wang and Dan Zhao, postdoctoral students, and Zia Ullah Khan, a doctoral student, finally discovered the right polymers. The team successfully created an electrolyte that has a higher capacity to transform heat into electric current, compared to the standard electrolytes that are used.
We still don’t know exactly why we’re getting this effect. But the fact is that we can convert and store 2,500 times more energy than the best of today’s supercapacitors linked to thermoelectric generators
Professor Xavier Crispin, Linköping University
The electrolyte includes simple, non-toxic, and low-cost materials that not only remain stable, but can also be handled under room temperature conditions. The thermoelectric supercapacitor powered by ions holds immense potential for storing solar electricity, to name just one single example. Two patents have resulted from this study. The team is hoping that this new study would pave the way for a new generation of energy storage that can be manufactured on a large scale.
Since 2014, the Knut and Alice Wallenberg Foundation has been supporting the study as part of the “Tail of the Sun” research project.